A) \[C{{O}_{2}}\]
B) \[S{{O}_{2}}\]
C) \[{{N}_{2}}O\]
D) \[CO\]
Correct Answer: B
Solution :
In \[\text{S}{{\text{O}}_{\text{2}}}\]molecule, S is \[\text{s}{{\text{p}}^{2}}\] \[-\]hybridized \[S(16)=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}},3{{p}^{4}}\]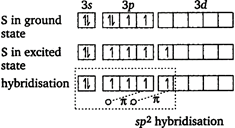 Note \[C{{O}_{2}},{{N}_{2}}O\]and CO, all are sp-hybridised.
Note \[C{{O}_{2}},{{N}_{2}}O\]and CO, all are sp-hybridised.
You need to login to perform this action.
You will be redirected in
3 sec
