A) \[10\sigma ,3\pi \]
B) \[10\sigma ,2\pi \]
C) \[9\sigma ,3\pi \]
D) \[10\sigma ,2\pi \]
Correct Answer: A
Solution :
Key Idea: (i) The first bond between any two atoms is sigma \[(\sigma )\]. (ii) Rest are pi \[(\pi )\] bonds. Draw the structure of compound and count the number of sigma and pi bonds in it.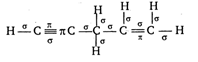 \[\therefore \] It has \[10\sigma \] and \[3\pi \] bonds
\[\therefore \] It has \[10\sigma \] and \[3\pi \] bonds
You need to login to perform this action.
You will be redirected in
3 sec
