A) \[SiC{{l}_{4}}\]
B) \[B{{F}_{4}}\]
C) \[N{{H}_{3}}\]
D) \[SeC{{l}_{4}}\]
Correct Answer: D
Solution :
Selenium tetrachloride can be shown as follows: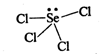 \[\text{(lp+}\sigma -bp=1+4=5\text{)}\] Hence, hybridization is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\text{.}\]Due to the presence of one lone pair of electron on selenium, its shape is distorted tetrahedral or see-saw structure.
\[\text{(lp+}\sigma -bp=1+4=5\text{)}\] Hence, hybridization is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\text{.}\]Due to the presence of one lone pair of electron on selenium, its shape is distorted tetrahedral or see-saw structure.
You need to login to perform this action.
You will be redirected in
3 sec
