A) the attack of \[\overline{O}H\] at the carbonyl group
B) the transfer of hydride ion to the carbonyl group
C) the abstraction of a proton from the carboxylic acid
D) the deprotonation of \[Ph-C{{H}_{2}}OH\]
Correct Answer: B
Solution :
Transfer of hydride ion to the carbonyl group is the slowest or the rate determining step.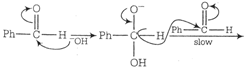
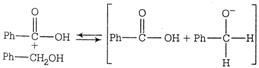
You need to login to perform this action.
You will be redirected in
3 sec
