A) one sp-sp sigma bond and two p-p pi bonds
B) two sp-sp sigma bonds and one p-p pi bond
C) one \[s{{p}^{2}}-s{{p}^{2}}\]sigma bond, one p-p pi bond
D) one \[s{{p}^{2}}-s{{p}^{2}}\]sigma bond, one \[s{{p}^{2}}-s{{p}^{2}}\]pi bond and one p-p pi bond
Correct Answer: D
Solution :
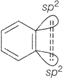 In benzene there is one \[s{{p}^{2}}-s{{p}^{2}}\sigma \]-bond, one \[s{{p}^{2}}-s{{p}^{2}},\pi \]-bond and \[p-p\pi \]-bond.
In benzene there is one \[s{{p}^{2}}-s{{p}^{2}}\sigma \]-bond, one \[s{{p}^{2}}-s{{p}^{2}},\pi \]-bond and \[p-p\pi \]-bond.
You need to login to perform this action.
You will be redirected in
3 sec
