A) \[Na+{{O}_{2}}\to Na{{O}_{2}}\xrightarrow{HCl\left( aq \right)}\] \[NaCl\xrightarrow{C{{O}_{2}}}N{{a}_{2}}C{{O}_{3}}\xrightarrow{\Delta }Na\]
B) \[Na\xrightarrow{{{O}_{2}}}N{{a}_{2}}O\xrightarrow{{{H}_{2}}O}NaOH\xrightarrow{C{{O}_{2}}}\] \[N{{a}_{2}}C{{O}_{3}}\xrightarrow{\Delta }Na\]
C) \[Na+{{H}_{2}}O\to NaOH\xrightarrow{HCl}NaCl\xrightarrow{C{{O}_{2}}}\] \[N{{a}_{2}}C{{O}_{3}}\xrightarrow{\Delta }Na\]?
D) \[Na+{{H}_{2}}O\to NaOH\xrightarrow{C{{O}_{2}}}N{{a}_{2}}C{{O}_{3}}\] \[\xrightarrow{HCl}\underset{\left( molten \right)}{\mathop{NaCl}}\,\xrightarrow{Electrolysis}Na\]
Correct Answer: D
Solution :
\[\tan \delta =\frac{1}{\sqrt{3}/2}\] \[=\frac{2}{\sqrt{3}}\] \[\delta ={{\tan }^{-1}}\left( \frac{2}{\sqrt{3}} \right)\]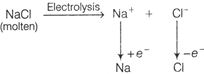
You need to login to perform this action.
You will be redirected in
3 sec
