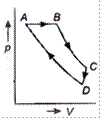
 Which of the following curves represents the equivalent cyclic process?
Which of the following curves represents the equivalent cyclic process?
A)
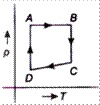
B)
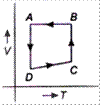
C)
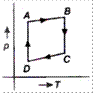
D)
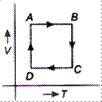
Correct Answer: A
Solution :
\[A\to B\to C\to D\to A\] is clockwise process. During \[A\to B,\] pressure is constant and \[B\to C,\] process follows \[P\propto \frac{1}{V},\] it means T is constant. During process \[C\to D,\], both p and V changes and process \[D\to A,\] follows \[P\propto \frac{1}{V},\] which means T is constant.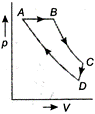 Hence, from above data it is clear that equivalent cyclic process is
Hence, from above data it is clear that equivalent cyclic process is 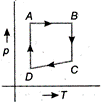
You need to login to perform this action.
You will be redirected in
3 sec
