question_answer 1) In the relation V = \[V=\frac{\pi p{{r}^{4}}}{8\eta l}\] where the symbols have their usual meanings, the dimensions of V are
A)
\[[{{M}^{0}}{{L}^{3}}{{T}^{0}}]\]
done
clear
B)
\[[{{M}^{0}}{{L}^{3}}{{T}^{-1}}]\]
done
clear
C)
\[[{{M}^{0}}{{L}^{-3}}T]\]
done
clear
D)
\[[M{{L}^{3}}{{T}^{0}}]\]
done
clear
View Answer play_arrow
question_answer 2) A point moves such that its displacement as a function of time is given by \[{{x}^{2}}={{t}^{2}}+1.\] Its acceleration at time t is
A)
\[\frac{1}{{{x}^{4}}}\]
done
clear
B)
\[\frac{t}{{{x}^{2}}}\]
done
clear
C)
\[\frac{t}{x}-\frac{{{t}^{2}}}{{{x}^{3}}}\]
done
clear
D)
\[\frac{1}{x}-\frac{t}{{{x}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 3) The angle of projection for which the horizontal range and the maximum height of the projectile are equal is
A)
\[45{}^\circ \]
done
clear
B)
\[\text{ }\!\!\theta\!\!\text{ =ta}{{\text{n}}^{-1}}(4)\]
done
clear
C)
\[\text{ }\!\!\theta\!\!\text{ =ta}{{\text{n}}^{-1}}(0.25)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 4) A body of mass 10 kg falls from a height of 5 m (g = 10 m/s) and is stopped within one-tenth of a second on the ground. The force of interaction is
A)
100 N
done
clear
B)
zero
done
clear
C)
1000 N
done
clear
D)
1100N
done
clear
View Answer play_arrow
question_answer 5) Two bodies, having masses in the ratio 1:4, have kinetic energies in the ratio 4:1. The ratio of their linear momentum is
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
2 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
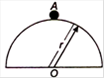
question_answer 6)
A mass m slides from rest down the surface of a frictionless hemispherical bowl of radius r from the highest point A. The velocity of mass when it reaches the bottom is
A)
\[\sqrt{2gr}\]
done
clear
B)
\[\sqrt{mgr}\]
done
clear
C)
2mgr
done
clear
D)
\[gr\]
done
clear
View Answer play_arrow
question_answer 7) An engine develops 20 HP. When rotating at a speed of 1800 rev/min. The torque that it delivers is
A)
400 N-m
done
clear
B)
60N-m
done
clear
C)
40 N-m
done
clear
D)
80 N-m
done
clear
View Answer play_arrow
question_answer 8) How much deep inside the earth (radius R) should a mango, so that his weight becomes one-fourth of that on earths surface?
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{R}{2}\]
done
clear
C)
\[\frac{3R}{4}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 9) If the speed of light were \[\frac{2}{3}\] of its present value, the energy released in a given atomic explosion will be decreased by a fraction
A)
\[\frac{2}{3}\]
done
clear
B)
\[\frac{4}{9}\]
done
clear
C)
\[\frac{5}{9}\]
done
clear
D)
\[\frac{2}{9}\]
done
clear
View Answer play_arrow
question_answer 10) 10. The difference between a photon and neutrino is
A)
the spin of photon is 1 and that of neutrino is \[\frac{1}{2}\] in units of \[\frac{h}{2\pi }\]
done
clear
B)
the spin of photon is \[\frac{1}{2}\] and that of neutrino is 1 in units of\[\frac{h}{2\pi }\]
done
clear
C)
both have equal spin but neutrino is electromagnetic in nature
done
clear
D)
both have unequal spin but neutrino is electromagnetic in nature
done
clear
View Answer play_arrow
question_answer 11) Alcohol is more volatile than water, because
A)
its boiling point is lower than water
done
clear
B)
it is an organic liquid
done
clear
C)
its freezing point is lower than water
done
clear
D)
its vapour pressure is 2.5 times greater than water
done
clear
View Answer play_arrow
question_answer 12) Certain substance emits wavelengths \[{{\lambda }_{1,}}{{\lambda }_{2,}}{{\lambda }_{3}}\] and \[{{\lambda }_{4,}}\] when it is at a high temperature. When the substance is at a colder temperature, it will absorb only the following wavelengths
A)
\[{{\lambda }_{1}}\]
done
clear
B)
\[{{\lambda }_{2}}\]
done
clear
C)
\[{{\lambda }_{1}}\,\text{and}\,{{\lambda }_{2}}\]
done
clear
D)
\[{{\lambda }_{1,}}{{\lambda }_{2,}}{{\lambda }_{3}}\,\text{and}\,{{\lambda }_{4}}\]
done
clear
View Answer play_arrow
question_answer 13) The deflection in a moving coil galvanometer is reduced to half when it is shunted with a coil of resistance S. The resistance of the galvanometer G = 40 \[\Omega .\] The value of Sis
A)
80\[\Omega .\]
done
clear
B)
40\[\Omega .\]
done
clear
C)
20\[\Omega .\]
done
clear
D)
15\[\Omega .\]
done
clear
View Answer play_arrow
question_answer 14) In Ingen-Hausz experiment the wax melts up to 5 cm and 10 cm on bars A and B respectively. The ratio of the thermal conductivities of A and B is
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
1:8
done
clear
D)
1 : 16
done
clear
View Answer play_arrow
question_answer 15) A body executes SHM with an amplitude A. Its energy is half kinetic and half potential when the displacement is
A)
\[\frac{A}{3}\]
done
clear
B)
\[\frac{A}{2}\]
done
clear
C)
\[\frac{A}{\sqrt{2}}\]
done
clear
D)
\[\frac{A}{2\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 16) The period of the simple pendulum in a stationary lift is T. If the lift move upwards with an acceleration g, the period will be
A)
\[\infty \]
done
clear
B)
\[\sqrt{\frac{3}{5}}T\]
done
clear
C)
\[\sqrt{\frac{5}{3}}T\]
done
clear
D)
\[\frac{T}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 17) A wave travelling along positive y-axis is given by\[y=A\text{ }sin\]\[(\omega -kx).\] If it is reflected from rigid boundary such that 80% amplitude is reflected, then equation of reflected wave is
A)
\[y=A\sin (\omega t+kx)\]
done
clear
B)
\[y=-0.8A\sin (\omega t+kx)\]
done
clear
C)
\[y=0.8A\sin (\omega t+kx)\]
done
clear
D)
\[y=A\sin (\omega t+0.8\,kx)\]
done
clear
View Answer play_arrow
question_answer 18) The surface charge density of the earth is
A)
\[{{10}^{-9}}c{{m}^{-2}}\]
done
clear
B)
\[{{10}^{-6}}c{{m}^{-2}}\]
done
clear
C)
\[-{{10}^{-9}}c{{m}^{-2}}\]
done
clear
D)
\[-100\text{ }c{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 19) The work done in increasing the voltage across the plates of a capacitor from 5V to 10 V is W. The work done in increasing the voltage from 10 V to 15V will be
A)
\[W\]
done
clear
B)
\[\frac{4W}{3}\]
done
clear
C)
\[\frac{5W}{3}\]
done
clear
D)
\[2W\]
done
clear
View Answer play_arrow
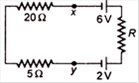
question_answer 20)
The current flowing in the given circuit is 0.1 A. The potential difference between the points \[x\] and y is
A)
4.0V
done
clear
B)
3.0V
done
clear
C)
2.5V
done
clear
D)
2.0V
done
clear
View Answer play_arrow
question_answer 21) The neutral temperature of a thermocouple is \[300{}^\circ C\]. What is the inversion temperature, if the temperature of cold junction is\[10{}^\circ C\]?
A)
\[590{}^\circ C\]
done
clear
B)
\[610{}^\circ C\]
done
clear
C)
\[310{}^\circ C\]
done
clear
D)
\[290{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 22) Two bulbs 100 W, 250 V and 200 W, 250 V are connected in parallel across a 500 V line. Then
A)
100 W bulb will be fused
done
clear
B)
200 W bulb will be fused
done
clear
C)
both bulbs will be fused
done
clear
D)
no bulb will be fused
done
clear
View Answer play_arrow
question_answer 23) An electron is moving in a circle of radius r in a uniform magnetic field B. Suddenly the field is D reduced to \[\frac{B}{2}.\] The radius of the circle now becomes
A)
\[\frac{r}{2}\]
done
clear
B)
\[\frac{r}{4}\]
done
clear
C)
2r
done
clear
D)
4r
done
clear
View Answer play_arrow
question_answer 24) If the number of turns per unit length of a coil of solenoid is doubled, the self-inductance of solenoid will be
A)
remain unchanged
done
clear
B)
be halved
done
clear
C)
be doubled
done
clear
D)
becomes four times
done
clear
View Answer play_arrow
question_answer 25) A copper ring is held horizontally and a bar magnet is dropped through the ring with its length along the axis of the ring. The acceleration of the falling magnet is
A)
equal to that due to gravity
done
clear
B)
less than that due to gravity
done
clear
C)
more than that due to gravity
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 26) An inductance, a capacitance and a resistance are connected in series across a source of alternating voltage. At resonance, the applied voltage and the current flowing through the circuit will have a phase difference
A)
\[\frac{\pi }{4}\]
done
clear
B)
zero
done
clear
C)
\[\pi \]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 27) To which region of electromagnetic spectrum, the frequency 1 GHz corresponding?
A)
Ultraviolet rays
done
clear
B)
Radiowaves
done
clear
C)
Visible radiation
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 28) When an object is moved along the axis of a lens, images three times the size of the object are obtained when the object is at 16 cm and at 8 cm respectively from the lens. The focal length and nature of the lens are
A)
12 cm, concave
done
clear
B)
4 cm, concave
done
clear
C)
12 cm, convex
done
clear
D)
4 cm, convex
done
clear
View Answer play_arrow
question_answer 29) Which one of the following phenomena is used in optical fibres?
A)
Scattering
done
clear
B)
Successive reflections
done
clear
C)
Refraction
done
clear
D)
Total internal reflection
done
clear
View Answer play_arrow
question_answer 30) The ratio of specific charge of a proton to that of an a-particle is
A)
1 : 4
done
clear
B)
1: 2
done
clear
C)
4: 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 31) A metallic surface ejects electrons when exposed to green light of intensity \[I\] but no photoelectrons are emitted when exposed to yellow light of intensity \[I\] It is possible to eject electrons from the same surface by
A)
yellow light of same intensity which is more than \[I\]
done
clear
B)
green light of any intensity
done
clear
C)
red light of any intensity
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 32) In hydrogen spectrum the wavelength of \[{{H}_{\alpha }}\] line is 656 nm, where as in the spectrum of a distant galaxy, \[{{H}_{\alpha }}\]line wavelength is 706 nm. Estimated speed of galaxy with respect to earth is
A)
\[2\times {{10}^{8}}m/s\]
done
clear
B)
\[2\times {{10}^{7}}m/s\]
done
clear
C)
\[2\times {{10}^{6}}m/s\]
done
clear
D)
\[2\times {{10}^{5}}m/s\]
done
clear
View Answer play_arrow
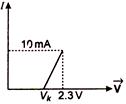
question_answer 33)
The resistance of a germanium junction diode, whose\[V-I\]is shown in figure is \[\text{(}{{\text{V}}_{\text{k}}}\text{=0}\text{.3}\,\text{V)}\]
A)
\[5\,k\,\Omega \]
done
clear
B)
\[0.2\,k\,\Omega \]
done
clear
C)
\[2.3\,k\,\Omega \]
done
clear
D)
\[\left( \frac{10}{2.3} \right)k\,\Omega \]
done
clear
View Answer play_arrow
question_answer 34) A circular loop of radius R carrying current \[I\] lies in \[xy\] plane with its centre at origin. The total magnetic flux through \[x-y\] plane is
A)
directly proportional to \[I\]
done
clear
B)
directly proportional to R
done
clear
C)
directly proportional to \[{{R}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 35) The essential distinction between X-rays and -\[y\]rays is that
A)
\[y\]-rays have smaller wavelength than -rays
done
clear
B)
\[y\]-rays emanate from nucleus while X-rays emanate from outer part of the atom
done
clear
C)
\[y\]-rays have greater ionizing power than X-rays
done
clear
D)
\[y\]-rays are more penetrating than X-rays
done
clear
View Answer play_arrow
question_answer 36) A convex mirror is used to form an image of a real object. Then, the incorrect statement is
A)
The image lies between the pole and the focus
done
clear
B)
The image is diminished in size
done
clear
C)
The image is erect
done
clear
D)
The image is real
done
clear
View Answer play_arrow
question_answer 37) A vessel is half-filled with a liquid of refractive index \[\mu \]. The other half of the vessel is filled with an immiscible liquid of refractive index 1.5\[\mu \]. The apparent depth of the vessel is 50% of the actual depth. Then \[\mu \] is
A)
1.4
done
clear
B)
1.5
done
clear
C)
1.6
done
clear
D)
1.67
done
clear
View Answer play_arrow
question_answer 38) Which of the following is not a unit of charge?
A)
Faraday
done
clear
B)
Frankline
done
clear
C)
Coulomb
done
clear
D)
Ampere/second
done
clear
View Answer play_arrow
question_answer 39) What is maximum height of a stone thrown vertically upward, if its velocity is halved in 1.5s? \[(g=10m/{{s}^{2}})\]
A)
20m
done
clear
B)
25m
done
clear
C)
30m
done
clear
D)
45m
done
clear
View Answer play_arrow
question_answer 40) In the Boolean algebra A B is same as
A)
\[\overline{A+B}\]
done
clear
B)
\[A.B\]
done
clear
C)
\[\overline{A.B}\]
done
clear
D)
\[A+B\]
done
clear
View Answer play_arrow
question_answer 41) Elements having the same number of nucleons and different number of protons are
A)
isotones
done
clear
B)
positrons
done
clear
C)
isotopes
done
clear
D)
isobars
done
clear
View Answer play_arrow
question_answer 42) Acetic acid exists in benzene solution in the dimeric form. In an actual experiment the vant Hoff factor was found to be 0.52. Then the degree of dissociation of acetic acid is
A)
0.48
done
clear
B)
0.88
done
clear
C)
0.96
done
clear
D)
0.52
done
clear
View Answer play_arrow
question_answer 43) The number of d-electron in\[F{{e}^{2+}}\](at. no. of \[Fe=26\]) is not equal to that of the
A)
p-electrons in Ne (at. no. =10)
done
clear
B)
s-electrons in Mg (at. no. =12)
done
clear
C)
d-electrons in Fe
done
clear
D)
p-electrons in\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 44) How many number of atoms are there in a cube based unit cell having one atom on each comer and two atoms on each body diagonal of cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 45) Which of the following substances has the highest melting point?
A)
\[NaCl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[MgO\]
done
clear
D)
\[BaO\]
done
clear
View Answer play_arrow
question_answer 46) Of the following reactions, only one is a redox reaction identify it.
A)
\[Ca{{(OH)}_{2}}+2HCl\xrightarrow[{}]{{}}CaC{{l}_{2}}+2{{H}_{2}}O\]
done
clear
B)
\[BaC{{l}_{2}}+MgS{{O}_{4}}\xrightarrow[{}]{{}}BaS{{O}_{4}}+MgC{{l}_{2}}\]
done
clear
C)
\[2{{S}_{2}}O_{7}^{2-}+{{H}_{2}}O\xrightarrow[{}]{{}}4SO_{4}^{2-}+4{{H}^{+}}\]
done
clear
D)
\[C{{u}_{2}}S+2FeO\xrightarrow[{}]{{}}2Cu+2Fe+S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 47) The Nemst equation giving dependence of electrode potential on concentration is
A)
\[E=E{}^\circ +\frac{2.303RT}{nF}\log \frac{[M]}{[{{M}^{n+}}]}\]
done
clear
B)
\[E=E{}^\circ +\frac{2.303RT}{nF}\log \frac{[{{M}^{n+}}]}{[M]}\]
done
clear
C)
\[E=E{}^\circ -\frac{2.303F}{nRT}\log \frac{[{{M}^{n+}}]}{[M]}\]
done
clear
D)
\[E=E{}^\circ -\frac{2.303RT}{nF}\log [{{M}^{n+}}]\]
done
clear
View Answer play_arrow
question_answer 48) At high pressures, the compressibility factor\[z\]is equal to
A)
unity
done
clear
B)
\[1-\frac{Pb}{RT}\]
done
clear
C)
\[1+\frac{Pb}{RT}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 49) A compound contains 69.5% oxygen and 30.5% nitrogen and its molecular weight is 92. The formula of the compound is
A)
\[{{N}_{2}}O\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 50) The reaction\[A\xrightarrow[{}]{{}}B\]follows first order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 h. What is the time taken for conversion of 0.9 mole of A to produce 0.675 mole of B?
A)
1 h
done
clear
B)
0.5 h
done
clear
C)
0.25 h
done
clear
D)
2h
done
clear
View Answer play_arrow
question_answer 51) Which order for ionisation energy is correct?
A)
\[Be<B<C<N<O\]
done
clear
B)
\[B<Be<C<O<N\]
done
clear
C)
\[Be>B>C>N>O\]
done
clear
D)
\[B<Be<N<C<O\]
done
clear
View Answer play_arrow
question_answer 52) How many grams of\[Ca{{C}_{2}}{{O}_{4}}\] will dissolve in distilled water to make one litre of saturated solution of it? (\[{{K}_{sp}}\] for\[Ca{{C}_{2}}{{O}_{4}}=2.5\times {{10}^{-9}}mo{{l}^{2}}{{L}^{-2}}\])
A)
0.0064 g
done
clear
B)
0.1028 g
done
clear
C)
0.1280 g
done
clear
D)
0.2056 g
done
clear
View Answer play_arrow
question_answer 53) In the extraction of copper from its sulphide ore, the metal is formed by reduction of \[C{{u}_{2}}O\]with
A)
\[FeS\]
done
clear
B)
CO
done
clear
C)
\[C{{u}_{2}}S\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 54) Which one of the following substances has the highest proton affinity?
A)
\[{{H}_{2}}S\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[P{{H}_{3}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 55) 1.520 g of the hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent weight of metal is
A)
1.520
done
clear
B)
0.995
done
clear
C)
19.00
done
clear
D)
9.00
done
clear
View Answer play_arrow
question_answer 56) Thermite is a mixture of
A)
Fe and\[Al\]
done
clear
B)
ferric oxide and aluminium powder
done
clear
C)
barium peroxide and magnesium powder
done
clear
D)
\[Cu\]and\[Al\]
done
clear
View Answer play_arrow
question_answer 57) A molecule\[X{{Y}_{2}}\]contains two a, two n bonds and one lone pair of electron in the valence shell of X. The arrangement of lone pair as well as bond pairs is
A)
square pyramidal
done
clear
B)
linear
done
clear
C)
trigonal planar
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 58) The maximum oxidation state exhibited by manganese (at. no. = 25) is
A)
+3
done
clear
B)
+4
done
clear
C)
+6
done
clear
D)
+7
done
clear
View Answer play_arrow
question_answer 59) Which of the following is a disaccharide?
A)
Cellobiose
done
clear
B)
Starch
done
clear
C)
Cellulose
done
clear
D)
Fructose
done
clear
View Answer play_arrow
question_answer 60) In the reaction of p-chlorotoluene with\[KN{{H}_{2}}\]in liq\[N{{H}_{3}},\]the major product is
A)
o-toluidine
done
clear
B)
m-toluidine
done
clear
C)
p-toluidine
done
clear
D)
p-chloroaniline
done
clear
View Answer play_arrow
question_answer 61) . Unstable substances exhibit higher radioactivity due to
A)
low p/n ratio
done
clear
B)
high p/n ratio
done
clear
C)
\[p/n=1\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 62) The pH of a dilute solution of acetic acid was found to be 4.3. The addition of a small crystal of sodium acetate will cause pH to
A)
become less than 4.3
done
clear
B)
become more than 4.3
done
clear
C)
remain equal to 4.3
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 63) Which of the following is paramagnetic?
A)
\[N{{H}_{2}}OH\]
done
clear
B)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[{{N}_{2}}{{H}_{6}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Sugar containing an impurity of common salt can be purified by crystallization from
A)
benzene
done
clear
B)
alcohol
done
clear
C)
petroleum ether
done
clear
D)
water
done
clear
View Answer play_arrow
question_answer 65) The\[C-H\]and\[C-C\]bond distance are shortest in
A)
methane
done
clear
B)
ethane
done
clear
C)
ethene
done
clear
D)
ethyne
done
clear
View Answer play_arrow
question_answer 66) Among the following identify the species with an atom in +6 oxidation state
A)
\[MnO_{4}^{-}\]
done
clear
B)
\[Cr(CN)_{6}^{3-}\]
done
clear
C)
\[NiF_{6}^{2-}\]
done
clear
D)
\[Cr{{O}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 67) Which of the following can effectively remove all types of hardness of water?
A)
Washing soda
done
clear
B)
Soap
done
clear
C)
Slaked lime
done
clear
D)
Boiling
done
clear
View Answer play_arrow
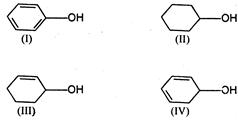
question_answer 68)
The increasing order of dehydration of following is
A)
\[I<II<II<IV\]
done
clear
B)
\[II<III<IV<I\]
done
clear
C)
\[I<III<II<IV\]
done
clear
D)
\[I<IV<II<III\]
done
clear
View Answer play_arrow
question_answer 69) The solution of Na in dry ether not only contains\[Na_{slov.}^{+}\]and\[e_{slov.}^{-}\]but also contains \[Na_{slov.}^{-}\]formed by the following disproportionatipn reaction. \[2Na(s)Na_{solv.}^{+}+Na_{solv.}^{-}\] The large sodide ion can be stabilized by making its
A)
cryptand-222
done
clear
B)
clatherate
done
clear
C)
complex with saticylaldehyde
done
clear
D)
complex with acetylacetone
done
clear
View Answer play_arrow
question_answer 70) The electronic configurations of the elements X, Y, Z and J are given below. Which element has the highest metallic character?
A)
\[X=2,8,4\]
done
clear
B)
\[Y=2,8,8\]
done
clear
C)
\[Z=2,8,8,1\]
done
clear
D)
\[J=2,8,8,7\]
done
clear
View Answer play_arrow
question_answer 71) Aniline reacts with\[NaN{{O}_{2}}\]and\[HCl\]at room temperature to give
A)
nitroaniline
done
clear
B)
phenol
done
clear
C)
chloroaniline
done
clear
D)
diazonium chloride
done
clear
View Answer play_arrow
question_answer 72) Which of the following is least soluble in water?
A)
HCHO
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) In the reaction \[C{{H}_{3}}CHO+HCN\xrightarrow[{}]{{}}C{{H}_{3}}CH(OH)CN\] A chiral centre is produced. This product would be
A)
laevo-rotatory
done
clear
B)
meso-compound
done
clear
C)
dextro-rotatory
done
clear
D)
racemic mixture
done
clear
View Answer play_arrow
question_answer 74) Which of the following is a primary standard in volumetric analysis?
A)
\[FeS{{O}_{4}}\]
done
clear
B)
\[FeS{{O}_{4}}{{(N{{H}_{4}})}_{2}}S{{O}_{4}}6{{H}_{2}}O\]
done
clear
C)
\[NaOH\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 75) In case of halides of\[S,Se,Te\]and\[Po,\]as the size of the halogen increases, the maximum coordination number of the element
A)
decreases
done
clear
B)
increases
done
clear
C)
remains unaffected
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 76) Which of the following has highest dipole moment?
A)
1,1-dichloroethene
done
clear
B)
cis-1, 2-dichloroethene
done
clear
C)
trans-1, 2-dichloroethene
done
clear
D)
All have same dipole moment
done
clear
View Answer play_arrow
question_answer 77) Presence of peroxides in old stocks of ethers can be tested by first treating them with\[F{{e}^{2+}}\]ions and then adding an aqueous solution of
A)
\[KCNS\]
done
clear
B)
\[HgC{{l}_{2}}\]
done
clear
C)
\[SnC{{l}_{2}}\]
done
clear
D)
\[KI\]
done
clear
View Answer play_arrow
question_answer 78) The decay constant of\[^{226}Ra\]is\[1.36\times {{10}^{-11}}{{s}^{-1}}\]. A sample of Ra- 226, having an activity of 1.5 \[mCi\]will contain
A)
\[4.0\times {{10}^{18}}atoms\]
done
clear
B)
\[205\times {{10}^{15}}atoms\]
done
clear
C)
\[3.5\times {{10}^{7}}atoms\]
done
clear
D)
\[4.7\times {{10}^{10}}atoms\]
done
clear
View Answer play_arrow
question_answer 79) Ammonia is obtained by the action of\[{{H}_{2}}O\]on
A)
\[CaC{{N}_{2}}\]
done
clear
B)
\[M{{g}_{3}}{{N}_{2}}\]
done
clear
C)
BN
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 80) Give the IUPAC name of the following \[C{{H}_{3}}\overset{\begin{smallmatrix} S \\ |\,\,| \end{smallmatrix}}{\mathop{C}}\,C{{H}_{2}}COOH\]is
A)
3-thiabutanoic acid
done
clear
B)
3-sulphobutanoic acid
done
clear
C)
3-thiobutanoic acid
done
clear
D)
3-thioxobutanoic acid
done
clear
View Answer play_arrow
question_answer 81) An alcohol on treatment with alkaline \[KMn{{O}_{4}}\]gives a brown precipitate, the alcohol is
A)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
B)
\[{{({{C}_{2}}{{H}_{5}})}_{3}}COH\]
done
clear
C)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}C(C{{H}_{3}})OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}CC{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 82) The compound, which on reaction with aqueous nitrous acid at low temperature, produces an oily nitrosoamine is
A)
diethylamine
done
clear
B)
ethylamine
done
clear
C)
aniline
done
clear
D)
methylamine
done
clear
View Answer play_arrow
question_answer 83) \[[Ag{{(N{{H}_{3}})}_{2}}OH]\]liberates silver when it reacts with
A)
\[HCOOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 84) Methyl n-propyl ketone on oxidation with \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] and\[{{H}_{2}}S{{O}_{4}}\] give mainly
A)
\[C{{H}_{3}}C{{H}_{2}}COOH+C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH+HCOOH\]
done
clear
C)
\[C{{H}_{3}}COOH+HCOOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH+C{{O}_{2}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 85) If\[KMn{{O}_{4}}\]is reduced by oxalic acid in an acidic medium, then oxidation number of Mn changes from
A)
4 to 2
done
clear
B)
6 to 4
done
clear
C)
+7 to +2
done
clear
D)
7 to 4
done
clear
View Answer play_arrow
question_answer 86) 0.01 M solution of\[{{H}_{2}}A\]has pH equal to 4, if \[{{K}_{{{a}_{1}}}}\]for the acid is\[4.45\times {{10}^{-7}},\]the concentration of\[H{{A}^{-}}\]ion in solution would be
A)
0.01 N
done
clear
B)
\[4.45\times {{10}^{-5}}\]
done
clear
C)
\[8.0\times {{10}^{-5}}\]
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 87) The percentage by weight of hydrogen in\[{{H}_{2}}{{O}_{2}}\]is
A)
5.88
done
clear
B)
6.25
done
clear
C)
25
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 88) A gas has a characteristic fishy odour and blue colour. It restores the colour of blackened lead paintings. It also bleaches in the absence of moisture. The gas is
A)
\[{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) A neutral compound with molecular formula \[{{C}_{3}}{{H}_{8}}O\]evolves\[{{H}_{2}}\]when treated with sodium metal and gives iodoform test. The compound is
A)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 90) In second group,\[{{H}_{2}}S\]is passed in the presence of dil.\[HCl\]because
A)
\[HCl\]checks incomplete precipitation of higher group radicals
done
clear
B)
\[HCl\]checks precipitation of sulphur
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 91) The rate of reaction of which of the following is not affected by pressure?
A)
\[PC{{l}_{3}}+C{{l}_{2}}\rightleftharpoons PC{{l}_{5}}\]
done
clear
B)
\[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\]
done
clear
C)
\[{{N}_{2}}+{{O}_{2}}\rightleftharpoons 2NO\]
done
clear
D)
\[S{{O}_{2}}+{{O}_{2}}\rightleftharpoons 2S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) Acidic buffer solution is produced on mixing the following aqueous solutions of
A)
\[N{{H}_{4}}Cl+N{{H}_{4}}OH\]
done
clear
B)
\[C{{H}_{3}}COONa+C{{H}_{3}}COOH\]
done
clear
C)
\[NaCl+NaOH\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 93) For an ideal gas, Joule-Thomson coefficient is
A)
zero
done
clear
B)
negative
done
clear
C)
positive
done
clear
D)
depend on molecular weight
done
clear
View Answer play_arrow
question_answer 94) Benzene on oxidation with\[{{V}_{2}}{{O}_{5}}\]produce
A)
toluene
done
clear
B)
benzaldehyde
done
clear
C)
maleic anhydride
done
clear
D)
benzophenone
done
clear
View Answer play_arrow
question_answer 95) The linkage present in proteins and peptides is
A)
\[-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\]
done
clear
B)
\[-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-O-O-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\]
done
clear
C)
\[-NH-\]
done
clear
D)
\[-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-NH\]
done
clear
View Answer play_arrow
question_answer 96) \[RCOCl+{{H}_{2}}\xrightarrow[{}]{Pd/BaS{{O}_{4}}}RCHO+HCl\] This reaction is known as
A)
Rosenmunds reaction
done
clear
B)
Stephens reaction
done
clear
C)
Meerwein Ponndorf verley reduction
done
clear
D)
Clemmensens reduction
done
clear
View Answer play_arrow
question_answer 97) Starch is a polymer of
A)
glucose
done
clear
B)
fructose
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 98) Ferric alum has the composition \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}.F{{e}_{2}}{{(S{{O}_{4}})}_{3}}.x{{H}_{2}}O\] The value of\[x\]is
A)
7
done
clear
B)
24
done
clear
C)
6
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 99) The use of plaster of Paris is in
A)
whitewash
done
clear
B)
toothpaste
done
clear
C)
black board chalk
done
clear
D)
surgery and dentistry
done
clear
View Answer play_arrow
question_answer 100) Nesslers reagent is used for the test of
A)
\[CrO_{4}^{2-}\]
done
clear
B)
\[MnO_{4}^{-}\]
done
clear
C)
\[NH_{4}^{+}\]
done
clear
D)
\[PO_{4}^{3-}\]
done
clear
View Answer play_arrow
question_answer 101) The book Genera plantarum was written by
A)
Bessey
done
clear
B)
Hutchinson
done
clear
C)
Engler and Pranti
done
clear
D)
Bentham and Hooker
done
clear
View Answer play_arrow
question_answer 102) Which of the following is not a true fish?
A)
Dog fish
done
clear
B)
Devil fish
done
clear
C)
Catfish
done
clear
D)
Sawfish
done
clear
View Answer play_arrow
question_answer 103) During mitosis, the number of chromosomes get
A)
change
done
clear
B)
no change
done
clear
C)
may be change if cell is mature
done
clear
D)
may be change if cell is immature
done
clear
View Answer play_arrow
question_answer 104) During transcription, the DNA site at which RNA polymerase binds is called
A)
promoter
done
clear
B)
regulator
done
clear
C)
receptor
done
clear
D)
enhancer
done
clear
View Answer play_arrow
question_answer 105) Bacteria do not possess
A)
capsule
done
clear
B)
ribosome
done
clear
C)
mitochondria
done
clear
D)
plasma membrane
done
clear
View Answer play_arrow
question_answer 106) Thermal cycler is used in this reaction
A)
radioactivity
done
clear
B)
enzyme catalysed
done
clear
C)
chemical reactions
done
clear
D)
PCR
done
clear
View Answer play_arrow
question_answer 107) Sporozoite, infectious stage of Plasmodium parasite contains
A)
two nucleus and a vacuole
done
clear
B)
one nucleus and several vacuoles
done
clear
C)
vacuoles and chloroplasts
done
clear
D)
a nucleus
done
clear
View Answer play_arrow
question_answer 108) The shape of chromosome is determined by
A)
centrosome
done
clear
B)
centromere
done
clear
C)
chromomere
done
clear
D)
telomere
done
clear
View Answer play_arrow
question_answer 109) Heteroneries stage is
A)
transformation of sexual individual into asexual
done
clear
B)
sexually matured with two regions atoke and epitoke
done
clear
C)
sexually immatured with two regions etoke and epitoke
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 110) In Funaria, calyptra is derived from
A)
capsule
done
clear
B)
columella
done
clear
C)
antheridium
done
clear
D)
archegonium
done
clear
View Answer play_arrow
question_answer 111) Biologically most resistant plant material is
A)
lignin
done
clear
B)
cutin
done
clear
C)
suberin
done
clear
D)
sporopollenin
done
clear
View Answer play_arrow
question_answer 112) Which of the following techniques other than microscopy is used for the study of cell ?
A)
Plasmolysis
done
clear
B)
Maceration
done
clear
C)
Chromatography
done
clear
D)
Auto-radiography
done
clear
View Answer play_arrow
question_answer 113) Earthworms have no special sense organs. Still they are sensitive to
A)
touch and sound
done
clear
B)
light and sound
done
clear
C)
touch, taste and sound
done
clear
D)
touch, taste and light
done
clear
View Answer play_arrow
question_answer 114) What will be the gametic chromosome number in a cell, if somatic cells have 40 chromosomes ?
A)
10
done
clear
B)
20
done
clear
C)
30
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 115) The ATP production in photosynthesis is called
A)
phototropism
done
clear
B)
phosphorylation
done
clear
C)
photooxidation
done
clear
D)
photophosphorylation
done
clear
View Answer play_arrow
question_answer 116) Duramen of heart wood is
A)
region of pericycle
done
clear
B)
another name of cambium
done
clear
C)
inner region of secondary wood
done
clear
D)
outer region of secondary wood
done
clear
View Answer play_arrow
question_answer 117) The term heterosis was first used by
A)
Shull
done
clear
B)
NEBorlaug
done
clear
C)
MS Swaminathan
done
clear
D)
RMishra
done
clear
View Answer play_arrow
question_answer 118) The larva of Bombyx mori is
A)
cocoon
done
clear
B)
nymph
done
clear
C)
caterpillar
done
clear
D)
trochophore
done
clear
View Answer play_arrow
question_answer 119) Gemmule formation in sponges is helpful in
A)
sexual reproduction
done
clear
B)
asexual reproduction
done
clear
C)
only dissemination
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 120) Organs not similar in structure and origin but perform the same function
A)
homologous structures
done
clear
B)
analogous structures
done
clear
C)
adaptive structures
done
clear
D)
variable structures
done
clear
View Answer play_arrow
question_answer 121) In Dryopteris, the opening mechanism of sporangium is effectively operated by
A)
stalk
done
clear
B)
annulus
done
clear
C)
stomium
done
clear
D)
peristome
done
clear
View Answer play_arrow
question_answer 122) Tunica corpus theory is used for
A)
shoot apex
done
clear
B)
root apex
done
clear
C)
leaf apex
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 123) The part of earth and atmosphere supporting life is
A)
biota
done
clear
B)
biome
done
clear
C)
ecotone
done
clear
D)
biosphere
done
clear
View Answer play_arrow
question_answer 124) Lendcels and hydathodes are small pores with which of the following common attributes?
A)
They allow exchange of gases
done
clear
B)
Their opening and closing is not regulated
done
clear
C)
They always remain closed
done
clear
D)
They are found on the same organ of plants
done
clear
View Answer play_arrow
question_answer 125) Chloragogen cells of earthworms are analogous to vertebrate
A)
lungs
done
clear
B)
liver
done
clear
C)
gut
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 126) Change in one base in mRNA leading to termination of polypeptide is known as which type of mutations?
A)
Sense
done
clear
B)
None-sense
done
clear
C)
Gibberish
done
clear
D)
Framshift
done
clear
View Answer play_arrow
question_answer 127) Taenia solium derives its energy from the breakdown of
A)
nucleic acids
done
clear
B)
amino acids
done
clear
C)
glycogen
done
clear
D)
glycerol
done
clear
View Answer play_arrow
question_answer 128) Which of the following hormone is produced during leaf fall ?
A)
ABA
done
clear
B)
Cytokinin
done
clear
C)
Florigen
done
clear
D)
Vernalin
done
clear
View Answer play_arrow
question_answer 129) Which fish selectively feed on larva of mosquito?
A)
Gambusia
done
clear
B)
Rohu
done
clear
C)
Glorias
done
clear
D)
Exocoetus
done
clear
View Answer play_arrow
question_answer 130) Lomentum is a term used to describe a kind of
A)
fruit
done
clear
B)
seed
done
clear
C)
inflorescence
done
clear
D)
outgrowth from seed
done
clear
View Answer play_arrow
question_answer 131) House-fly possesses
A)
two pairs of wings
done
clear
B)
one pair of wings
done
clear
C)
three pairs of wings
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 132) Sunken stomata are found in
A)
Nerium
done
clear
B)
Hydrilla
done
clear
C)
mango
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 133) Which Fish has an electric organ?
A)
Torpedo
done
clear
B)
Rhinoceros
done
clear
C)
Scoliodon
done
clear
D)
Cat fish
done
clear
View Answer play_arrow
question_answer 134) Three most abundant elements in protoplasm are
A)
carbon, sodium and hydrogen
done
clear
B)
carbon, hydrogen and \[{{O}_{2}}\]
done
clear
C)
nitrogen, carbon and \[{{O}_{2}}\]
done
clear
D)
carbon, phosphorus and oxygen
done
clear
View Answer play_arrow
question_answer 135) Genetically engineered bacteria is used for the production of
A)
thyroxin
done
clear
B)
human insulin
done
clear
C)
epinephrine
done
clear
D)
cortisol
done
clear
View Answer play_arrow
question_answer 136) Inulin is a
A)
lipid
done
clear
B)
protein
done
clear
C)
human insulin
done
clear
D)
polysaccharide
done
clear
View Answer play_arrow
question_answer 137) Jumping genes in maize were discovered by
A)
Hugo de Vries
done
clear
B)
TH Morgan
done
clear
C)
Barbara McClintock
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 138) Which of the following is not a pentose sugar?
A)
arabinose
done
clear
B)
xylose
done
clear
C)
xylulose
done
clear
D)
mannose
done
clear
View Answer play_arrow
question_answer 139) Nucleolus is the site for the synthesis of
A)
DNA
done
clear
B)
mRNA
done
clear
C)
tRNA
done
clear
D)
ribosomes
done
clear
View Answer play_arrow
question_answer 140) Which of the following statement is not true ?
A)
Animal cells never contain chloroplast unlike many plant cells
done
clear
B)
Animal cells contain ultrascopic chloroplast while plant cells contain microscopic chloroplast
done
clear
C)
Plant cells have cellulosic cell wall while animal cells do not
done
clear
D)
Plant cells have central vacuoles while animal-cells do not
done
clear
View Answer play_arrow
question_answer 141) Which of the following factor controls the human population density ?
A)
Industry
done
clear
B)
Climate
done
clear
C)
Communication
done
clear
D)
Natural resources
done
clear
View Answer play_arrow
question_answer 142) Chromosomal aberration is due to
A)
aneuploidy
done
clear
B)
polyploidy
done
clear
C)
physical effects
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 143) What used to described as Nissi granules in a nerve cell are now identified as
A)
cell metabolites
done
clear
B)
fat granules
done
clear
C)
ribosomes
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 144) Ageing of an animal body is associated with deterioration in its
A)
connective tissue
done
clear
B)
glandular tissue
done
clear
C)
epithelial tissue
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 145) Photochemical smog always contains
A)
\[{{O}_{3}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[\text{P}{{\text{O}}_{\text{4}}}\]
done
clear
View Answer play_arrow
question_answer 146) The posterior end of Ancylostoma has
A)
anus
done
clear
B)
curved tail
done
clear
C)
bursa
done
clear
D)
caudal spine
done
clear
View Answer play_arrow
question_answer 147) Quantasomes are found in
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
lysosome
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 148) In Pinus, male cone bears a large number of
A)
ligules
done
clear
B)
anthers
done
clear
C)
microsporophylls
done
clear
D)
megasporophylls
done
clear
View Answer play_arrow
question_answer 149) Hydroponics is a
A)
airless culture
done
clear
B)
waterless culture
done
clear
C)
soilless culture
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 150) Lysosomes are formed by
A)
endoplasmic reticulum
done
clear
B)
mitochondrion
done
clear
C)
Golgi membrane
done
clear
D)
both [a] and [c]
done
clear
View Answer play_arrow
question_answer 151) Nissls granules are characteristic of
A)
muscle tissue
done
clear
B)
connective tissue
done
clear
C)
nerve tissue
done
clear
D)
epithelial tissue
done
clear
View Answer play_arrow
question_answer 152) Gastric vacuoles are analogous to the
A)
peroxisomes
done
clear
B)
alimentary canal of higher animals
done
clear
C)
mouth of higher animals
done
clear
D)
liver of higher animals
done
clear
View Answer play_arrow
question_answer 153) Genetic information is carried by long chain macromolecules made up of
A)
amino acids
done
clear
B)
nucleotides
done
clear
C)
chromosomes
done
clear
D)
enzymes
done
clear
View Answer play_arrow
question_answer 154) Fertilization of ovum in human being occur in
A)
fallopian tube
done
clear
B)
cervix
done
clear
C)
fundus
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 155) Which one are correctely matched?
A)
Vitamin-E-tocopherol
done
clear
B)
Vitamin-D-riboflavin
done
clear
C)
Vitamin-B-calciferol
done
clear
D)
Vitamin-A-thiamine
done
clear
View Answer play_arrow
question_answer 156) Believers in spontaneous generation theory assumed that
A)
organisms arose only from other similar organisms
done
clear
B)
organisms could arise only from air
done
clear
C)
organisms arose-from non-living material
done
clear
D)
organisms always arise from air
done
clear
View Answer play_arrow
question_answer 157) Half-life period of \[{{C}^{14}}\]is
A)
500 years
done
clear
B)
5000 years
done
clear
C)
50 years
done
clear
D)
\[5\times {{10}^{4}}years\]
done
clear
View Answer play_arrow
question_answer 158) Maximum number of plasmids discovered so far
A)
50 kilo base
done
clear
B)
500 kilo base
done
clear
C)
5000 kilo base
done
clear
D)
5 kilo base
done
clear
View Answer play_arrow
question_answer 159) The lytic enzymes released by sperm is
A)
acrosome
done
clear
B)
ligase
done
clear
C)
androgamone
done
clear
D)
hyaluronidase
done
clear
View Answer play_arrow
question_answer 160) Transfer of genes from one gene pool to another is called
A)
speciation
done
clear
B)
genetic drift
done
clear
C)
gene flow
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 161) A material which arrests cell division, is obtained from
A)
Crocus
done
clear
B)
Colchicum
done
clear
C)
Dalbergia
done
clear
D)
Chrysanthemum
done
clear
View Answer play_arrow
question_answer 162) Chromosomes in a bacterial cell can be 1-3 in number and
A)
are always circular
done
clear
B)
are always linear
done
clear
C)
can be either circular or linear, but never both within the same cell
done
clear
D)
can be circular as well as linear within the same cell
done
clear
View Answer play_arrow
question_answer 163) Example of an aggregate fruit is
A)
Pyrus mains
done
clear
B)
Ananas sarivus
done
clear
C)
Annona squamosal
done
clear
D)
Arcocarpiis integrifolia
done
clear
View Answer play_arrow
question_answer 164) Identify the edible freshwater teleosts
A)
sharks
done
clear
B)
rays and skates
done
clear
C)
Hilsa hilsa
done
clear
D)
Catla catla
done
clear
View Answer play_arrow
question_answer 165) The blood cell which shows phagocytosis is
A)
monocyte
done
clear
B)
barsophil
done
clear
C)
eosinophil
done
clear
D)
platelets
done
clear
View Answer play_arrow
question_answer 166) In angiosperms, which of the following part is triploid ?
A)
Zygote
done
clear
B)
Endosperm
done
clear
C)
Archegonia
done
clear
D)
Coralloid root
done
clear
View Answer play_arrow
question_answer 167) Mitochondria are often seen aggregated around
A)
food vacuole
done
clear
B)
contractile vacuole
done
clear
C)
water vacuole
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 168) Addisons disease results from
A)
hypertrophy of gonad
done
clear
B)
hyposecretion of adrenal cortex
done
clear
C)
hyperactivity of leydig cells
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 169) Main difference between active and passive transport across cell membrane is that
A)
active transport occurs more rapidly than passive
done
clear
B)
passive transport is non-selective
done
clear
C)
passive transport is confined to anions and cations
done
clear
D)
passive transport requires a concentration gradient across the membrane, whereas active transport requires metabolic energy
done
clear
View Answer play_arrow
question_answer 170) Which statement is correct for muscle- contraction ?
A)
Length of H zone decreases
done
clear
B)
Length of A band decreases
done
clear
C)
Length of I band remain constant
done
clear
D)
Length of two Z line increases
done
clear
View Answer play_arrow
question_answer 171) Aleurone layer helps in
A)
growth
done
clear
B)
nutrition
done
clear
C)
protection
done
clear
D)
water absorption
done
clear
View Answer play_arrow
question_answer 172) Internal cork disease in apple is due to the deficiency of
A)
iron
done
clear
B)
copper
done
clear
C)
boron
done
clear
D)
phosphorus
done
clear
View Answer play_arrow
question_answer 173) When an animal turns sideways to the Sun to get the maximum amount of Sun on its flanks, this is an example of:
A)
motivation
done
clear
B)
habitation
done
clear
C)
posture
done
clear
D)
orientation
done
clear
View Answer play_arrow
question_answer 174) The main components of plasma membrane are
A)
lipids and proteins
done
clear
B)
lipids, proteins and carbohydrates
done
clear
C)
lipids only
done
clear
D)
proteins only
done
clear
View Answer play_arrow
question_answer 175) Pollination by insects is called
A)
zoophily
done
clear
B)
chiropterophily
done
clear
C)
anemophily
done
clear
D)
entomophily
done
clear
View Answer play_arrow
question_answer 176) The genetic material in \[\phi \times 174\] is
A)
single stranded DNA
done
clear
B)
single stranded RNA
done
clear
C)
double stranded DNA
done
clear
D)
double stranded RNA
done
clear
View Answer play_arrow
question_answer 177) The pH of stomach ranges between
A)
7.1 to 8.2
done
clear
B)
7.6 to 8.6
done
clear
C)
1.5 to 2.0
done
clear
D)
6.8 to 7.2
done
clear
View Answer play_arrow
question_answer 178) Removal of ring wood of tissue outside the vascular cambium from the tree trunk kills it because
A)
water cannot move up
done
clear
B)
food does not travel down and roots become starved
done
clear
C)
shoot become starved
done
clear
D)
annual ring are not produced
done
clear
View Answer play_arrow
question_answer 179) The high amount of E. coli in water is an indicator of
A)
hardness of water
done
clear
B)
industrial pollution
done
clear
C)
sewage pollution
done
clear
D)
presence of chlorine in water
done
clear
View Answer play_arrow
question_answer 180) Which of the following connects glycolysis to Krebs cycle?
A)
Acetyl Co-A
done
clear
B)
Ribozyme
done
clear
C)
Cytochrome oxidase
done
clear
D)
N-acetyl glucosamine
done
clear
View Answer play_arrow
question_answer 181) Which of the following theory explain structure of protoplasm?
A)
Surface tension theory
done
clear
B)
Colloidal theory
done
clear
C)
Sol-gel theory
done
clear
D)
Viscosity theory
done
clear
View Answer play_arrow
question_answer 182) An insect without metamorphosis is
A)
silver Fish
done
clear
B)
cray fish
done
clear
C)
bedbug
done
clear
D)
pediculus
done
clear
View Answer play_arrow
question_answer 183) Lichens show
A)
mutualism
done
clear
B)
commensalism
done
clear
C)
parasitism
done
clear
D)
saprophytism
done
clear
View Answer play_arrow
question_answer 184) In which of the following animal anal tail is found?
A)
Earthworm
done
clear
B)
Lower invertebrates
done
clear
C)
Scorpion
done
clear
D)
Snake
done
clear
View Answer play_arrow
question_answer 185) Which of the following statements is true
A)
All enzymes are proteins
done
clear
B)
All proteins are enzymes
done
clear
C)
All enzymes are not proteins
done
clear
D)
All enzymes and hormones are proteins
done
clear
View Answer play_arrow
question_answer 186) This is not the cell of areolar tissue
A)
macrophages
done
clear
B)
Schwann cell
done
clear
C)
plasma cell
done
clear
D)
adipose cell
done
clear
View Answer play_arrow
question_answer 187) Anaerobic respiration after glycolysis is called
A)
restoration
done
clear
B)
fermentation
done
clear
C)
multiplication
done
clear
D)
fragmentation
done
clear
View Answer play_arrow
question_answer 188) Portuguese man of war is
A)
Obelia
done
clear
B)
Pennatula
done
clear
C)
Coral
done
clear
D)
Physalia
done
clear
View Answer play_arrow
question_answer 189) In photosynthesis, photolysis of water is useful for
A)
reduction of NADP
done
clear
B)
oxidation of NADP
done
clear
C)
oxidation of FAD
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 190) Liquid food drinking is
A)
imbibition
done
clear
B)
pinocytosis
done
clear
C)
phagocytosis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 191) Terminating codons are also called
A)
initiating codons
done
clear
B)
stop signals
done
clear
C)
central dogma
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 192) A pome fruit is said to be false because
A)
the pericarp is inconspicuous
done
clear
B)
the endocarp is cartilagenous
done
clear
C)
fruit is derived from superior ovary
done
clear
D)
fruit is present in fleshy edible thalamus
done
clear
View Answer play_arrow
question_answer 193) In photosynthesis, the light energy is utilized in
A)
converting \[ATP\] into \[ADP\]
done
clear
B)
converting \[\text{C}{{\text{H}}_{\text{3}}}\] into \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}\]
done
clear
C)
converting \[\text{ADP}\] into \[\text{ATP}\]
done
clear
D)
converting \[\text{C}{{\text{O}}_{\text{2}}}\] into carbohydrate
done
clear
View Answer play_arrow
question_answer 194) Receptors of sensation produced when a person eats red chillies are located on which part of tongue ?
A)
Tip
done
clear
B)
Base
done
clear
C)
Side
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 195) In which of the following haemocyanin pigment is found ?
A)
Annelida
done
clear
B)
Echinodermata
done
clear
C)
Insects
done
clear
D)
Lower invertebrates
done
clear
View Answer play_arrow
question_answer 196) Photosystem-II occurs in
A)
grana
done
clear
B)
stroma
done
clear
C)
entire chloroplast.
done
clear
D)
chloroplast membrane
done
clear
View Answer play_arrow
question_answer 197) When a cluster of genes show linkage behaviour they
A)
do not show a chromosome map
done
clear
B)
show recombination during meiosis
done
clear
C)
do not show independent assortment
done
clear
D)
induce cell division
done
clear
View Answer play_arrow
question_answer 198) What is the function of centrosome ?
A)
Cell division
done
clear
B)
Cell plate formation
done
clear
C)
Cell differentiation
done
clear
D)
Cell wall formation
done
clear
View Answer play_arrow
question_answer 199) Keratinization of the skin is prevented by
A)
vitamin-A
done
clear
B)
vitamin-B
done
clear
C)
vitamin-C
done
clear
D)
vitamin-D
done
clear
View Answer play_arrow
question_answer 200) Christmas disease is another name for
A)
haemophilia-B
done
clear
B)
hepatitis-B
done
clear
C)
Downs syndrome
done
clear
D)
sleeping sickness
done
clear
View Answer play_arrow