A) \[C{{l}_{2}}\]
B) \[{{O}_{2}}\]
C) \[{{N}_{2}}\]
D) \[H{{e}_{2}}\]
Correct Answer: B
Solution :
A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair. In Oxygen molecule\[({{O}_{2}}),\]two oxygen atoms achieve stable structures by sharing two pairs of electrons as shown below. The double bond is shown conventionally by two lines joining the atoms. Each line represents one pair of shared electrons.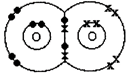 or \[O=O\]
or \[O=O\]
You need to login to perform this action.
You will be redirected in
3 sec
