A) Axial P-Cl bonds are longer than equatorial P-Cl bonds
B) \[PC{{l}_{5}}\] molecule is non-reactive
C) Three equatorial P-Cl bonds make an angle of \[120{}^\circ \] with each other
D) Two axial P-Cl bonds make an angle of \[180{}^\circ \]with each other
Correct Answer: B
Solution :
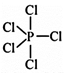 Three equatorial bonds and two axial bonds. Due to unsymmetry \[PC{{l}_{5}}\] is reactive.
Three equatorial bonds and two axial bonds. Due to unsymmetry \[PC{{l}_{5}}\] is reactive.
You need to login to perform this action.
You will be redirected in
3 sec
