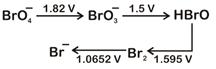
 |
| Then the species undergoing disproportionation is |
A) \[\text{B}{{\text{r}}_{2}}\]
B) \[\text{BrO}_{\text{4}}^{\text{-}}\]
C) \[\text{BrO}_{\text{3}}^{\text{-}}\]
D) \[\text{HBrO}\]
Correct Answer: D
Solution :
\[\overset{+1}{\mathop{\text{HBrO}}}\,\xrightarrow{{}}\overset{0}{\mathop{\text{B}{{\text{r}}_{\text{2}}}}}\,,E_{HBrO/B{{r}_{2}}}^{O}=1.595V\] \[\overset{+1}{\mathop{\text{HBrO}}}\,\xrightarrow{{}}\overset{+5}{\mathop{\text{BrO}_{3}^{-}}}\,,E_{BrO_{3}^{-}/HBrO}^{O}=1.5V\] \[\text{E}_{\text{cell}}^{\text{o}}\] for the disproportionation of \[\text{HBrO}\], \[\text{E}_{\text{cell}}^{\text{o}}\text{-E}_{\text{HBrO/B}{{\text{r}}_{\text{2}}}}^{\text{o}}\text{-E}_{\text{BrO}_{\text{3}}^{\text{-}}\text{/HBrO}}^{\text{o}}\] \[\text{=1}\text{.595-1}\text{.5}\] \[\text{=0}\text{.095V=+ve}\] Hence, option is correct Sol..You need to login to perform this action.
You will be redirected in
3 sec
