A) Square planar geometry and paramagnetic
B) Tetrahedral geometry and diamagnetic
C) Square planar geometry and diamagnetic
D) Tetrahedral geometry and paramagnetic
Correct Answer: B
Solution :
\[\text{Ni(28): }\!\![\!\!\text{ Ar }\!\!]\!\!\text{ 3}{{\text{d}}^{\text{8}}}\text{4}{{\text{s}}^{\text{2}}}\] \[\because \text{CO}\] is a strong field ligand Configuration would be :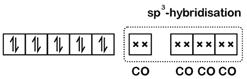 For, four ?CO?-ligands hybridisation would be \[\text{s}{{\text{p}}^{\text{3}}}\] and thus the complex would be diamagnetic and of tetrahedral geometry.
For, four ?CO?-ligands hybridisation would be \[\text{s}{{\text{p}}^{\text{3}}}\] and thus the complex would be diamagnetic and of tetrahedral geometry. 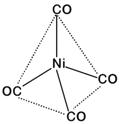
You need to login to perform this action.
You will be redirected in
3 sec
