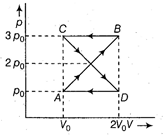
A) \[{{p}_{0}}{{V}_{0}}\]
B) \[2{{p}_{0}}{{V}_{0}}\]
C) \[\frac{{{p}_{0}}{{V}_{0}}}{2}\]
D) zero
Correct Answer: D
Solution :
Work done in the cyclic process = Area bound by the closed configuration = Area of closed configuration = \[\left\{ 2\left[ \frac{1}{2}({{v}_{0}}/2)\times {{p}_{0}} \right]+\left\{ -2\left[ \frac{1}{2}({{v}_{0}}/2){{p}_{0}} \right] \right. \right\}\] = zero.You need to login to perform this action.
You will be redirected in
3 sec
