A) \[\text{Te}{{\text{F}}_{\text{2}}}\]
B) \[lCl_{2}^{-}\]
C) \[SbC{{l}_{3}}\]
D) \[\text{BaC}{{\text{l}}_{\text{2}}}\]
Correct Answer: B
Solution :
Species having the same number of bond pairs and lone pairs are is structural (have same structure).| Species | lp + bp | Structure |
| \[Xe{{F}_{2}}\] | \[4lp+2bp\] | Linear |
| \[Te{{F}_{2}}\] | \[2lp+2bp\] | Angular or V-shape |
| \[lCl_{2}^{-}\] | \[4lp+2bp\] | Linear 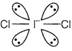 |
| \[BaC{{l}_{2}}\] | \[0lp+2bp\] | Cl-Ba-Cl (linear) |
You need to login to perform this action.
You will be redirected in
3 sec
