A) \[{{e}^{3}}t_{2}^{3}\]
B) \[{{e}^{4}}t_{2}^{2}\]
C) \[t_{2g}^{4}e_{g}^{2}\]
D) \[t_{2g}^{6}e_{g}^{0}\]
Correct Answer: D
Solution :
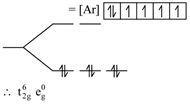
| [d] \[{{K}_{4}}\left[ Fe{{(CN)}_{6}} \right]\] |
| has \[F{{e}^{+2}}=\text{ }\!\![\!\!\text{ }Ar\text{ }\!\!]\!\!\text{ }3{{d}^{6}}\] |
 |
You need to login to perform this action.
You will be redirected in
3 sec
