A) \[\Delta S=0\]
B) \[\Delta G=0\]
C) \[\Delta H=0\]
D) \[\Delta H=\Delta G=\Delta S=0\]
Correct Answer: C
Solution :
| [c] Energy profile diagram for a reaction is as From the figure, it is clear that |
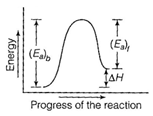 |
| \[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}+\Delta H\] |
| [Here \[{{({{E}_{a}})}_{b}}=\] activation energy of forward reaction]. |
| If \[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}\] |
| then \[\Delta H=0\] |
You need to login to perform this action.
You will be redirected in
3 sec
