A) Hydrogen bonding
B) Dipole-dipole interaction
C) Covalent bonds
D) London dispersion force
Correct Answer: A
Solution :
| Key Idea When H atom is directly linked with N or O, or F, inter or intermolecular H-bonding is formed. |
| In between \[C{{H}_{3}}OH\] molecules intermolecular H-bonding exist. |
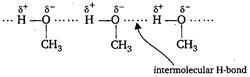 |
| Hence, it is the intermolecular H-bonding that must be overcome in converting liquid \[C{{H}_{3}}OH\] to gas. |
You need to login to perform this action.
You will be redirected in
3 sec
