A) the electro negativity of F is greater than that of O
B) \[{{H}_{2}}O\] involves hydrogen bonding whereas\[Be{{F}_{2}}\] is a discrete molecule
C) \[{{H}_{2}}O\]is linear and \[Be{{F}_{2}}\] is angular
D) \[{{H}_{2}}O\] is angular and \[Be{{F}_{2}}\] is linear
Correct Answer: D
Solution :
| The structure of \[{{H}_{2}}O\] is angular V-shape and as\[s{{p}^{3}}\]-hybridisation and bond angle is\[105{}^\circ \]. Its dipole moment value is positive or more than zero. |
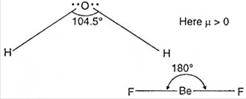 |
| but in \[Be{{F}_{2}}\], structure is linear due to sp-hybridization\[(\mu =0)\] |
| Thus, due to \[\mu >0,\,{{H}_{2}}O\]is dipolar and due to\[\mu =0,\]\[Be{{F}_{2}}\] is non-polar. |
You need to login to perform this action.
You will be redirected in
3 sec
