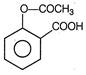
 Aspirin is a pain reliever with\[p{{K}_{a}}=2\]. Two tablets each containing 0.09 g of aspirin are dissolved in 100 mL solution. pH will be
Aspirin is a pain reliever with\[p{{K}_{a}}=2\]. Two tablets each containing 0.09 g of aspirin are dissolved in 100 mL solution. pH will be
A) 0.5
B) 1.0
C) 0.8
D) 2.0
Correct Answer: D
Solution :
Aspirin is a weak acid\[=0.09\times 2\text{ }g/100\text{ }mL\] \[=1.8g\,{{L}^{-1}}\] \[\frac{1.8}{180}mol\,{{L}^{-1}}=0.01\,M\] pH (weak acid) \[=\frac{1}{2}[p{{K}_{a}}-\log C]=\frac{1}{2}(2+2)=2\]You need to login to perform this action.
You will be redirected in
3 sec
