A) \[\frac{\pi }{4}\]
B) \[f(x)=x{{e}^{-x}}\]
C) \[[0,\infty ),\]
D) \[0\]
Correct Answer: A
Solution :
\[u={{e}^{x}}\sin x\]ion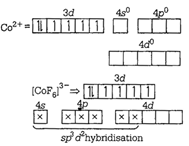 \[v={{e}^{x}}\cos x\]is a weak ligand. It cannot pair up d-electrons and forms outer orbital octahedral complex. \[v\frac{du}{dx}-u\frac{dv}{dx}={{u}^{2}}+{{v}^{2}}\]arid\[\frac{{{d}^{2}}u}{d{{x}^{2}}}=2v\] are strong ligands. So, they pair up d-electrons and form their inner orbital complex. \[\frac{{{d}^{2}}u}{d{{x}^{2}}}=-2u\]ion
\[v={{e}^{x}}\cos x\]is a weak ligand. It cannot pair up d-electrons and forms outer orbital octahedral complex. \[v\frac{du}{dx}-u\frac{dv}{dx}={{u}^{2}}+{{v}^{2}}\]arid\[\frac{{{d}^{2}}u}{d{{x}^{2}}}=2v\] are strong ligands. So, they pair up d-electrons and form their inner orbital complex. \[\frac{{{d}^{2}}u}{d{{x}^{2}}}=-2u\]ion 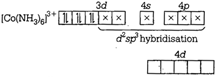 \[{{\tan }^{-1}}\left( \frac{\sqrt{1+{{x}^{2}}}-1}{x} \right)\]ion
\[{{\tan }^{-1}}\left( \frac{\sqrt{1+{{x}^{2}}}-1}{x} \right)\]ion 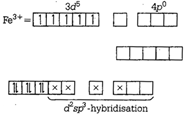 (d)\[{{\tan }^{-1}}\left( \frac{2x\sqrt{1-{{x}^{2}}}}{1-2{{x}^{2}}} \right)\]ion
(d)\[{{\tan }^{-1}}\left( \frac{2x\sqrt{1-{{x}^{2}}}}{1-2{{x}^{2}}} \right)\]ion 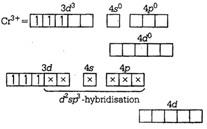
You need to login to perform this action.
You will be redirected in
3 sec
