A)
![]()
B)

C)
![]()
D)
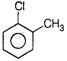
Correct Answer: C
Solution :
Nucleophile always attacks on electron deficient site. Presence of electron withdrawing group such as \[\frac{\pi }{6}\]etc., decreases the electron-density on benzene nucleous, hence such group activates the ring towards nucleophilic attack. While presence of electron releasing groups such as R or OR increase the electron density, thus deactivate the nucleus toward nucleophilie attack. \[\frac{\pi }{2}\]group activate the ring more than Cl toward nucleophilic attack hence react readily with nucleophile.You need to login to perform this action.
You will be redirected in
3 sec
