A) \[B{{F}_{3}}\]
B) \[N{{H}_{3}}\]
C) \[C{{H}_{2}}C{{l}_{2}}\]
D) \[S{{O}_{3}}\]
Correct Answer: A
Solution :
\[B{{F}_{3}}\] has zero dipole-moment as it is a planar molecule with F?B?F bond angle \[120{}^\circ \]. Hence, bond moments cancel each other. The resultant dipole moment becomes zero.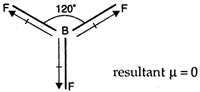
You need to login to perform this action.
You will be redirected in
3 sec
