A) dibasic acid
B) tribasic acid
C) neutral
D) monobasic acid
Correct Answer: D
Solution :
Hypophosphorus acid\[({{H}_{3}}P{{O}_{2}})\]is a monobasic acid as it gives only one type of salt\[(Na{{H}_{2}}P{{O}_{2}})\]. \[{{H}_{3}}P{{O}_{2}}\rightleftharpoons {{H}^{+}}+{{H}_{2}}PO_{2}^{-}\] In phosphorus oxy-acids, the H-atoms attached to O-atom, are ionisable while P?H bonded H-atoms are not ionisable and are reducing. Hypophosphorus acid contains only one ?OH group and have 2 (P?H) bonded H-atoms, as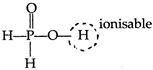
You need to login to perform this action.
You will be redirected in
3 sec
