A) only \[sp\text{-}\]hybridised carbon atoms
B) both \[sp\] and \[sp\text{-}\]hybridised atoms
C) only \[s{{p}^{2}}\text{-}\]hybridised carbon atoms
D) \[sp,s{{p}^{2}}\] and \[s{{p}^{3}}\text{-}\]hybridised carbon atoms
Correct Answer: D
Solution :
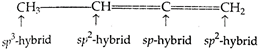 Carbon atom, whose valencies are satisfied with 4 single bonds, is \[s{{p}^{3}}\text{-}\]hybrid. Carbon atom, whose valencies are satisfied with one double-bond, is \[s{{p}^{2}}\text{-}\]hybrid while the carbon atom, whose valencies are satisfied either by 2 double-bonds or one triple and one single bond, is \[sp\text{-}\]hybrid.
Carbon atom, whose valencies are satisfied with 4 single bonds, is \[s{{p}^{3}}\text{-}\]hybrid. Carbon atom, whose valencies are satisfied with one double-bond, is \[s{{p}^{2}}\text{-}\]hybrid while the carbon atom, whose valencies are satisfied either by 2 double-bonds or one triple and one single bond, is \[sp\text{-}\]hybrid.
You need to login to perform this action.
You will be redirected in
3 sec
