A) \[1.68\times {{10}^{6}}J\]
B) \[1.71\times {{10}^{7}}J\]
C) \[1.51\times {{10}^{5}}J\]
D) 1\[1.67\times {{10}^{5}}J\]
Correct Answer: D
Solution :
| [d] |
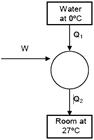 |
| heat required to freeze 5 kg water |
| |
| |
| |
| for can nots cycle |
| |
| |
| |
| |
| |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
