A)
B)
C)
D)
Correct Answer: D
Solution :
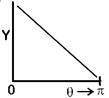
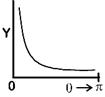
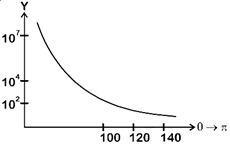
| [d] In 1911, Ernest Rutherford publish a formula. |
| Which indicates that number of particles (Y) that would be deflected by an angle \[\theta \] due to scattering is |
| \[{{Y}_{(\theta )}}=\frac{K}{{{\sin }^{4}}\frac{\theta }{2}}\] Where K = constant |
| \[\therefore \] Corresponding graph. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
