A) \[{{\lambda }_{1}}={{\lambda }_{2}}=4{{\lambda }_{3}}=9{{\lambda }_{4}}\]
B) \[{{\lambda }_{1}}=2{{\lambda }_{2}}=3{{\lambda }_{3}}=4{{\lambda }_{4}}\]
C) \[4{{\lambda }_{1}}=2{{\lambda }_{2}}=2{{\lambda }_{3}}={{\lambda }_{4}}\]
D) \[{{\lambda }_{1}}=2{{\lambda }_{2}}=2{{\lambda }_{3}}={{\lambda }_{4}}\]
Correct Answer: A
Solution :
[a] 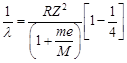 |
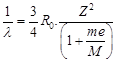 |
| [M = mass of mucleus] |
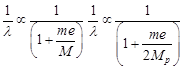 |
| [ |
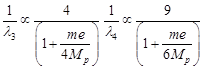 |
| If |
You need to login to perform this action.
You will be redirected in
3 sec
