A) \[\left( \frac{e}{2m} \right)\frac{{{\operatorname{n}}^{2}}h}{2\pi }\]
B) \[\left( \frac{e}{m} \right)\frac{\operatorname{n}h}{2\pi }\]
C) \[\left( \frac{e}{2m} \right)\frac{\operatorname{n}h}{2\pi }\]
D) \[\left( \frac{e}{m} \right)\frac{{{\operatorname{n}}^{2}}h}{2\pi }\]
Correct Answer: C
Solution :
| [c] As, |
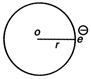 |
| and magnetic moment |
| |
| |
| Now, |
It becomes, 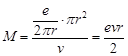 (ii)
(ii) |
| Also, |
| |
| Putting this value in Eq. (ii), we get |
| |
You need to login to perform this action.
You will be redirected in
3 sec
