A) \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\text{and}\,{{[Fe{{({{H}_{2}}O)}_{4}}]}^{2-}}\]
B) \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\text{and}\,{{[Co(CoC{{l}_{4}})]}^{2-}}\]
C) \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\text{and}\,{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
D) \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\text{and}\,Cr{{({{H}_{2}}O)}_{6}}{{]}^{2+}}\]
Correct Answer: C
Solution :
In option (1) : \[{{[CoC{{l}_{4}}]}^{2-}},C{{o}^{2+}}(3{{d}^{7}})\] with W.F.L.,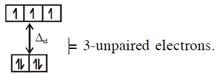 \[\And {{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},F{{e}^{2+}}(3{{d}^{6}})\]with W.F.L.,
\[\And {{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},F{{e}^{2+}}(3{{d}^{6}})\]with W.F.L., 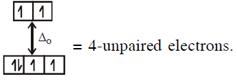 In option (2) : \[[Cr{{(Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L.,
In option (2) : \[[Cr{{(Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L., 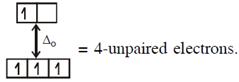 \[{{[CoC{{l}_{4}}]}^{2-}}C{{o}^{2+}}(3{{d}^{7}})\]with W.F.L.,
\[{{[CoC{{l}_{4}}]}^{2-}}C{{o}^{2+}}(3{{d}^{7}})\]with W.F.L., 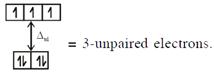 In option (3) : \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L.,
In option (3) : \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L., 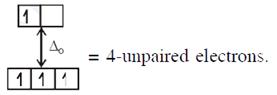 \[\And {{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},F{{e}^{2+}}(3{{d}^{6}})\] with W.F.L.,
\[\And {{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},F{{e}^{2+}}(3{{d}^{6}})\] with W.F.L., 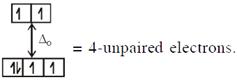 Here both complexes have same unpaired electrons i.e. = 4 In option (4) : \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}},M{{n}^{2+}}(3{{d}^{5}})\] with
Here both complexes have same unpaired electrons i.e. = 4 In option (4) : \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}},M{{n}^{2+}}(3{{d}^{5}})\] with 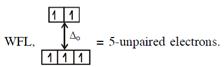 \[\And {{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L.,
\[\And {{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}},C{{r}^{2+}}(3{{d}^{4}})\]with W.F.L., 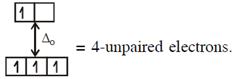
You need to login to perform this action.
You will be redirected in
3 sec
