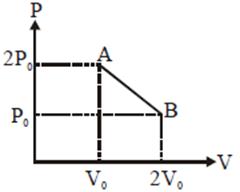
 [JEE Main Solved Paper-2016 ]
[JEE Main Solved Paper-2016 ]
A) \[\frac{9{{P}_{0}}{{V}_{0}}}{nR}\]
B) \[\frac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
C) \[\frac{3{{P}_{0}}{{V}_{0}}}{2nR}\]
D) \[\frac{9{{P}_{0}}{{V}_{0}}}{2nR}\]
Correct Answer: B
Solution :
T will be max where product of PV is max.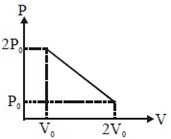 equation of line\[P=\frac{-{{P}_{0}}}{{{V}_{0}}}V+3{{P}_{0}}\] \[PV=\frac{-{{P}_{0}}}{{{V}_{0}}}{{V}^{2}}+3{{P}_{0}}V=x\](says) \[\left. \begin{align} & \frac{dx}{dV}=0\Rightarrow V=\frac{3{{V}_{0}}}{2} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Rightarrow P=\frac{3{{P}_{0}}}{2} \\ \end{align} \right\}\] here PV product is max. \[\Rightarrow \]\[T=\frac{PV}{nR}=\frac{9}{4}\frac{{{P}_{0}}{{V}_{0}}}{nR}\] Alternate
equation of line\[P=\frac{-{{P}_{0}}}{{{V}_{0}}}V+3{{P}_{0}}\] \[PV=\frac{-{{P}_{0}}}{{{V}_{0}}}{{V}^{2}}+3{{P}_{0}}V=x\](says) \[\left. \begin{align} & \frac{dx}{dV}=0\Rightarrow V=\frac{3{{V}_{0}}}{2} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Rightarrow P=\frac{3{{P}_{0}}}{2} \\ \end{align} \right\}\] here PV product is max. \[\Rightarrow \]\[T=\frac{PV}{nR}=\frac{9}{4}\frac{{{P}_{0}}{{V}_{0}}}{nR}\] Alternate 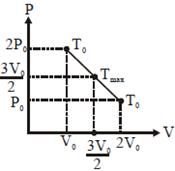 Since initial and final temperature are equal hence maximum temperature is at middle of line. PV = nRT \[\frac{\left( \frac{3{{P}_{0}}}{2} \right)\left( \frac{3{{P}_{0}}}{2} \right)}{nR}={{T}_{\max }}.\Rightarrow \frac{9{{P}_{0}}{{V}_{0}}}{4nR}={{T}_{\max }}.\]
Since initial and final temperature are equal hence maximum temperature is at middle of line. PV = nRT \[\frac{\left( \frac{3{{P}_{0}}}{2} \right)\left( \frac{3{{P}_{0}}}{2} \right)}{nR}={{T}_{\max }}.\Rightarrow \frac{9{{P}_{0}}{{V}_{0}}}{4nR}={{T}_{\max }}.\]
You need to login to perform this action.
You will be redirected in
3 sec
