A) 2 lines in the Lyman series and I line in the Balmar series
B) 3 lines in the Lyman series
C) 1 line in the Lyman series and 2 lines in the balmar series
D) 3 lines in the balmer series
Correct Answer: A
Solution :
\[E=\frac{hc}{\lambda }\Rightarrow \lambda =\frac{hc}{E}=\frac{6.62\times {{10}^{-34}}\times 3\times {{10}^{8}}}{12.5\times 1.6\times {{10}^{-19}}}\] \[=993{\AA}\] \[\frac{1}{\lambda }=R\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\] (where Rydberg constant, \[R=1.097\times {{10}^{7}}\]) or\[\frac{1}{993\times {{10}^{-10}}}=1.097\times {{10}^{7}}\left( \frac{1}{{{1}^{2}}}-\frac{1}{n_{2}^{2}} \right)\] Solving we get \[{{n}_{2}}=3\]Spectral lines Total number of spectral lines =3 Two lines in Lyman series for \[{{n}_{1}}=1,{{n}_{2}}=2\]and \[{{n}_{1}}=1,{{n}_{2}}=3\] and one in Balmer series for \[{{n}_{1}}=2,{{n}_{2}}=3\]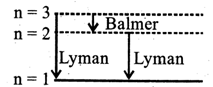
You need to login to perform this action.
You will be redirected in
3 sec
