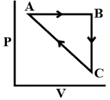
 \[\Delta {{\text{U}}_{BC}}=-5mJ\,\,mo{{l}^{-1}},\,\,{{q}_{AB}}=2kJ\,\,mo{{l}^{-1}}\] \[{{W}_{AB}}=-5\,\,kJ\,\,mo{{l}^{-1}},\,\,{{W}_{CA}}=3\,\,kJ\,\,mo{{l}^{-1}}\] Heat absorbed by the system during process CA is:
\[\Delta {{\text{U}}_{BC}}=-5mJ\,\,mo{{l}^{-1}},\,\,{{q}_{AB}}=2kJ\,\,mo{{l}^{-1}}\] \[{{W}_{AB}}=-5\,\,kJ\,\,mo{{l}^{-1}},\,\,{{W}_{CA}}=3\,\,kJ\,\,mo{{l}^{-1}}\] Heat absorbed by the system during process CA is:
A) \[18kJ\,\,mo{{l}^{-1}}\]
B) \[-18kJ\,\,mo{{l}^{-1}}\]
C) \[-5\,\,kJ\,\,mo{{l}^{-1}}\]
D) \[+5\,\,kJ\,\,mo{{l}^{-1}}\]
Correct Answer: D
Solution :
\[\Delta {{U}_{AB}}={{q}_{AB}}+{{W}_{AB}}=2+(-5)=-3kJ/mol\] \[\Delta {{U}_{BC}}=-5kJ/mol\] For cyclic process, \[\Delta U=0\] \[\Delta {{U}_{AB}}+\Delta {{U}_{BC}}+\Delta {{U}_{CA}}=0\] \[\Delta {{U}_{CA}}=-\Delta {{U}_{AB}}-\Delta {{U}_{BC}}\] \[\Delta {{U}_{CA}}=-(-3)-(-5)=8kJ/mol\] \[\Delta {{U}_{CA}}={{q}_{CA}}+{{W}_{CA}}\] \[8={{q}_{CA}}+3\] \[{{q}_{CA}}=+5kJ/mol\] Heat absorbed has positive sign.You need to login to perform this action.
You will be redirected in
3 sec
