A) \[\text{I}{{\text{F}}_{\text{7}}}\] : pentagonal bipyramid
B) \[\text{Br}{{\text{F}}_{5}}\] : trigonal bipyramid
C) \[\text{Br}{{\text{F}}_{3}}\] : planar T-shaped
D) \[\text{IC}{{\text{I}}_{3}}\]: planar dimeric
Correct Answer: B
Solution :
The molecular geometry of \[\text{B}{{\text{r}}_{2}}{{F}_{5}}\] is square pyramidal with asymmetric charge distribution on the central atom.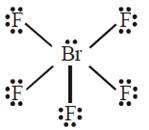
You need to login to perform this action.
You will be redirected in
3 sec
