A) \[2KMn{{O}_{4}}\to {{K}_{2}}Mn{{O}_{4}}+Mn{{O}_{2}}+{{O}_{2}}\]
B) \[2MnO_{4}^{-}+10{{I}^{-}}+16{{H}^{+}}\to 2M{{n}^{2+}}+5{{I}_{2}}+8{{H}_{2}}O\]
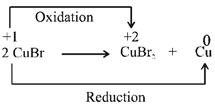
C) \[2CuBr\to CuB{{r}_{2}}+Cu\]
D) \[2NaBr+C{{l}_{2}}\to 2NaCl+B{{r}_{2}}\]
Correct Answer: C
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
