question_answer 1) A long spring is stretched by \[2\,\,cm\]. Its potential energy is \[U\]. If the spring is stretched by \[10\,\,cm,\] the potential energy stored in it will be:
A)
\[\frac{U}{25}\]
done
clear
B)
\[\frac{U}{5}\]
done
clear
C)
\[3U\]
done
clear
D)
\[25U\]
done
clear
View Answer play_arrow
question_answer 2) The coefficient of restitution e for a perfectly elastic collision is:
A)
\[1\]
done
clear
B)
zero
done
clear
C)
infinite
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 3) A soap bubble is given negative charge. Then its radius:
A)
decreases
done
clear
B)
increases
done
clear
C)
remains unchanged
done
clear
D)
nothing can be said because sufficient information is not available
done
clear
View Answer play_arrow
question_answer 4) Source of energy in sun is caused by:
A)
fusion of heavy nuclei
done
clear
B)
fission of heavy nuclei
done
clear
C)
fusion of hydrogen nuclei
done
clear
D)
fission of hydrogen nuclei
done
clear
View Answer play_arrow
question_answer 5) A particle moves along a circular path under the action of a force. The work done by the force is:
A)
positive and non-zero
done
clear
B)
negative and non-zero
done
clear
C)
zero
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 6) A particle is moving in a circle with uniform speed. It has constant:
A)
velocity
done
clear
B)
acceleration
done
clear
C)
kinetic energy
done
clear
D)
displacement
done
clear
View Answer play_arrow
question_answer 7) If\[|\overset{\to }{\mathop{\mathbf{A}}}\,+\overset{\to }{\mathop{\mathbf{B}}}\,|=|\overset{\to }{\mathop{\mathbf{A}}}\,-\overset{\to }{\mathop{\mathbf{B}}}\,|\],\[A\] and \[B\] are finite then:
A)
\[\overset{\to }{\mathop{\mathbf{A}}}\,\] is parallel to \[\overset{\to }{\mathop{\mathbf{B}}}\,\]
done
clear
B)
\[\overset{\to }{\mathop{\mathbf{A}}}\,\] is anti-parallel to \[\overset{\to }{\mathop{\mathbf{B}}}\,\]
done
clear
C)
\[\overset{\to }{\mathop{\mathbf{A}}}\,\] and \[\overset{\to }{\mathop{\mathbf{B}}}\,\] are equal in magnitude
done
clear
D)
\[\overset{\to }{\mathop{\mathbf{A}}}\,\] and \[\overset{\to }{\mathop{\mathbf{B}}}\,\] are mutually perpendicular
done
clear
View Answer play_arrow
question_answer 8) A number of small drops of mercury adiabatically coalesce to form a single drop. The temperature of the drop:
A)
increases
done
clear
B)
remains same
done
clear
C)
decreases
done
clear
D)
depend on size
done
clear
View Answer play_arrow
question_answer 9) Light waves can be polarised as they are:
A)
transverse
done
clear
B)
of high frequency
done
clear
C)
longitudinal
done
clear
D)
reflected
done
clear
View Answer play_arrow
question_answer 10) In which process the speed of transfer of heat is maximum?
A)
Conduction
done
clear
B)
Convection
done
clear
C)
Radiation
done
clear
D)
In all these heat is transferred with the same speed
done
clear
View Answer play_arrow
question_answer 11) Young's experiment establishes that:
A)
light consists of waves
done
clear
B)
light consists of particles
done
clear
C)
light is neither of particles nor waves
done
clear
D)
light consists of particles and waves both
done
clear
View Answer play_arrow
question_answer 12) A heating coil is labelled \[100\,\,W,\,\,\text{ }220\,\,V\]. The coil is cut into two equal pieces and the two pieces are joined in parallel to the same source. The energy now liberated per second is:
A)
\[400\,\,J\]
done
clear
B)
\[25\,\,J\]
done
clear
C)
\[50\,\,J\]
done
clear
D)
\[200\,\,J\]
done
clear
View Answer play_arrow
question_answer 13) Bending of light rays around the edges of an obstacle is known as:
A)
refraction
done
clear
B)
polarization
done
clear
C)
diffraction
done
clear
D)
reflection
done
clear
View Answer play_arrow
question_answer 14) A convex lens of focal length \[40\,\,cm\] is in contact with a concave lens of focal length\[25\,\,cm\]. The power of the combination of the two lenses is:
A)
\[-1.5\,\,D\]
done
clear
B)
\[-6.5\,\,D\]
done
clear
C)
\[6.5\,\,D\]
done
clear
D)
\[6.67\,\,D\]
done
clear
View Answer play_arrow
question_answer 15) Particle \[A\] moves in \[S\text{-}N\] direction and particle \[B\] moves in \[W\text{-}E\] direction. Then the .velocity of particle \[A\] with respect to \[B\] is:
A)
\[N\text{-}W\] direction
done
clear
B)
\[N\text{-}E\] direction
done
clear
C)
\[S\text{-}W\] direction
done
clear
D)
\[S\text{-}E\]direction
done
clear
View Answer play_arrow
question_answer 16) Weightlessness experienced while orbiting the earth in space ships is the result of:
A)
inertia
done
clear
B)
acceleration
done
clear
C)
zero gravity
done
clear
D)
centre of gravity
done
clear
View Answer play_arrow
question_answer 17) If one mole of a monoatomic gas \[(\gamma =5/3)\] is mixed with one mole of a diatomic gas \[(\gamma =7/5)\], the value of \[\gamma \] for the mixture is:
A)
\[1.40\]
done
clear
B)
\[1.50\]
done
clear
C)
\[1.53\]
done
clear
D)
\[3.07\]
done
clear
View Answer play_arrow
question_answer 18) Which of the following statements is wrong?
A)
Sound travels in straight line
done
clear
B)
Sound travels as waves
done
clear
C)
Sound is a form of energy
done
clear
D)
Sound travels faster in vacuum than in air
done
clear
View Answer play_arrow
question_answer 19) If momentum of a certain body is increased by \[50%\], then increases in the KE of the body will be:
A)
\[25%\]
done
clear
B)
\[50%\]
done
clear
C)
\[100%\]
done
clear
D)
\[125%\]
done
clear
View Answer play_arrow
question_answer 20) A uniform chain of length \[L\] and mass \[M\] is lying on a smooth table and one-third of its length is hanging vertically down over the edge of the table. If \[g\] is the acceleration due to gravity the work required to pull the hanging pan of the chain on the table is:
A)
\[MgL\]
done
clear
B)
\[\frac{1}{3}MgL\]
done
clear
C)
\[\frac{1}{9}MgL\]
done
clear
D)
\[\frac{1}{18}MgL\]
done
clear
View Answer play_arrow
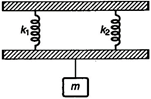
question_answer 21)
In the arrangement shown in the figure for vertical oscillations of the mass \[m\], the period is:
A)
\[T=2\pi \sqrt{\frac{m}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
C)
\[T=2\pi \sqrt{\frac{m({{k}_{1}}+{{k}_{2}})}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{mg}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
View Answer play_arrow
question_answer 22) A person completes half of its journey with speed vi and rest half with speed \[{{v}_{2}}\]. The average speed of the person is:
A)
\[v=\frac{1}{2}({{v}_{1}}+{{v}_{2}})\]
done
clear
B)
\[v=\frac{2{{v}_{1}}{{v}_{2}}}{{{v}_{1}}+{{v}_{2}}}\]
done
clear
C)
\[v=\frac{{{v}_{1}}{{v}_{2}}}{{{v}_{1}}+{{v}_{2}}}\]
done
clear
D)
\[v=\sqrt{{{v}_{1}}{{v}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 23) A particle moves along a straight line such that its displacement at any time \[t\] is given by\[s={{t}^{3}}-3{{t}^{2}}+2m\]. The displacement when the acceleration becomes zero is:
A)
\[zero\]
done
clear
B)
\[2\,\,m\]
done
clear
C)
\[3\,\,m\]
done
clear
D)
\[-2\,\,m\]
done
clear
View Answer play_arrow
question_answer 24) \[[M{{L}^{2}}{{T}^{-3}}]\] represents the dimensions of:
A)
pressure
done
clear
B)
energy
done
clear
C)
power
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 25) The time period of a simple pendulum in a lift descending with constant acceleration \[g\] is:
A)
\[T=2\pi \sqrt{\frac{l}{8}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{l}{2g}}\]
done
clear
C)
zero
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 26) A transverse wave is given by\[y=A\sin 2\pi \left( \frac{t}{T}-\frac{x}{\lambda } \right)\]. The maximum particle, velocity is equal to 4 times the wave velocity, when:
A)
\[\lambda =2\pi A\]
done
clear
B)
\[\lambda =\frac{1}{2}\pi A\]
done
clear
C)
\[\lambda =\pi \,\,A\]
done
clear
D)
\[\lambda =\frac{1}{4}\pi A\]
done
clear
View Answer play_arrow
question_answer 27) A tuning fork gives \[4\] beats with \[50\,\,cm\] length of a sonometer wire. If the length of the wire is shortened by \[1\,\,cm\] the number of beats is still the same. The frequency of the fork is:
A)
\[396\,\,Hz\]
done
clear
B)
\[400\,\,Hz\]
done
clear
C)
\[404\,\,Hz\]
done
clear
D)
\[384\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 28) Electron volt is a unit of:
A)
potential
done
clear
B)
charge
done
clear
C)
power
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 29) Light of frequency \[v\] is incident on a certain photoelectric substance with threshold frequency \[{{v}_{0}}\]. The work function for the substance is:
A)
\[hv\]
done
clear
B)
\[h{{v}_{0}}\]
done
clear
C)
\[h(v-{{v}_{0}})\]
done
clear
D)
\[h(v+{{v}_{0}})\]
done
clear
View Answer play_arrow
question_answer 30) For principal quantum number \[n=3\] the possible values of orbital quantum number\[l\]are:
A)
\[1,\,\,2,\,\,3\]
done
clear
B)
\[0,\,\,1,\,\,2,\,\,3\]
done
clear
C)
\[0,\,\,1,\,\,2\]
done
clear
D)
\[-1,\,\,0,\,\,+1\]
done
clear
View Answer play_arrow
question_answer 31) Certain radioactive substance reduces to \[25%\] of its value in \[16\] days. Its half-life is:
A)
32 days
done
clear
B)
8 days
done
clear
C)
64 days
done
clear
D)
28 days
done
clear
View Answer play_arrow
question_answer 32) Penetrating power of \[X-\]rays does not depend on:
A)
wavelength
done
clear
B)
energy
done
clear
C)
potential difference
done
clear
D)
current in the filament
done
clear
View Answer play_arrow
question_answer 33) The ratio of resistance for forward to reverse bias of \[p-n\] junction is:
A)
\[{{10}^{2}}:1\]
done
clear
B)
\[{{10}^{-2}}:1\]
done
clear
C)
\[1:{{10}^{-4}}\]
done
clear
D)
\[1:{{10}^{4}}\]
done
clear
View Answer play_arrow
question_answer 34) If a current flows through an infinitely long straight wire, the magnetic field produced at a point \[1\,\,m\] away from it, is:
A)
\[2\times {{10}^{-3}}T\]
done
clear
B)
\[2\times {{10}^{-1}}T\]
done
clear
C)
\[2\times {{10}^{-7}}T\]
done
clear
D)
\[2\pi \times {{10}^{-6}}T\]
done
clear
View Answer play_arrow
question_answer 35)
Two circular discs \[A\] and \[B\] with equal radii are blackened. They are heated to same temperature and are cooled under identical conditions. What inference do you draw from their cooling curves?
A)
\[A\] and \[B\] have same specific heats
done
clear
B)
Specific heat of \[A\] is less
done
clear
C)
Specific heat of \[B\] is less
done
clear
D)
Nothing can be said
done
clear
View Answer play_arrow
question_answer 36) When a charged particle, travelling with uniform speed enters a uniform magnetic field perpendicularly then, its kinetic energy:
A)
remains constant
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 37) A circular coil of radius \[4\,\,cm\] and number of turns \[20\] carries a current of \[3\,\,A\]. It is placed in a magnetic field of \[0.5\,\,T\]. The magnetic dipole moment of the coil is:
A)
\[0.60\,\,A{{m}^{2}}\]
done
clear
B)
\[0.45\,\,A{{m}^{2}}\]
done
clear
C)
\[0.30\,\,A{{m}^{2}}\]
done
clear
D)
\[0.15\,\,A{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 38) In a circuit 5% of total current passes through a galvanometer. If resistance of the galvanometer is \[G\], then value of the shunt is:
A)
\[19\,\,G\]
done
clear
B)
\[20\,\,G\]
done
clear
C)
\[G/20\]
done
clear
D)
\[G/19\]
done
clear
View Answer play_arrow
question_answer 39) In a moving coil galvanometer, deflection \[\phi \] and current \[I\] flowing through it are related by:
A)
\[I\propto \tan \phi \]
done
clear
B)
\[I\propto \phi \]
done
clear
C)
\[I\propto {{\phi }^{2}}\]
done
clear
D)
\[I\propto 1/\phi \]
done
clear
View Answer play_arrow
question_answer 40) A solenoid of length \[l\] metre has self-inductance \[L\] henry. If number of turns are doubled, its self-inductance:
A)
remains same
done
clear
B)
becomes \[2L\] henry
done
clear
C)
becomes \[4L\] henry
done
clear
D)
becomes \[\frac{L}{\sqrt{2}}\] henry
done
clear
View Answer play_arrow
question_answer 41) The voltage of an AC supply varies with time \[(t)\] as \[V=120\sin \,\,100\pi t\cos \,\,100\pi t\]. The maximum voltage and frequency respectively are:
A)
\[120\,\,V,\,\,100\,\,Hz\]
done
clear
B)
\[120/\sqrt{2}V,\,\,100\,\,Hz\]
done
clear
C)
\[60\,\,V,\,\,200\,\,Hz\]
done
clear
D)
\[60\,\,V,\,\,100\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 42) Two point charges of \[+3\mu C\] and \[-3\mu C\] are at a distance\[2\times 10\,\,m\] apart from each other. The electric field at a distance of \[0.6\,\,m\] from the dipole in broadside-on position is:
A)
\[150\,\,N{{C}^{-1}}\]
done
clear
B)
\[250\,\,N{{C}^{-1}}\]
done
clear
C)
\[60\,\,N{{C}^{-1}}\]
done
clear
D)
\[35\,\,N{{C}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 43) Two parallel large thin metal sheets have equal surface charge densities \[(\sigma =26.4\times {{10}^{-12}}C/{{m}^{2}})\] of opposite signs. The electric field between these sheets is:
A)
\[1.5\,\,N/C\]
done
clear
B)
\[1.5\times {{10}^{-10}}N/C\]
done
clear
C)
\[3\,\,N/C\]
done
clear
D)
\[3\times {{10}^{-10}}N/C\]
done
clear
View Answer play_arrow
question_answer 44) \[N\] identical spherical drops are charged to the same potential \[V\]. They combine to form a bigger drop. The potential of the big drop will be:
A)
\[V{{N}^{1/3}}\]
done
clear
B)
\[V{{N}^{2/3}}\]
done
clear
C)
\[V\]
done
clear
D)
\[VN\]
done
clear
View Answer play_arrow
question_answer 45) Three condensers of capacitance \[2\mu F\] each are connected in series. The resultant capacitance is:
A)
\[6\mu F\]
done
clear
B)
\[3/2\mu F\]
done
clear
C)
\[2/3\mu F\]
done
clear
D)
\[5\mu F\]
done
clear
View Answer play_arrow
question_answer 46) Two electric bulbs rated \[{{P}_{1}}\] watt, \[V\] volt and \[{{P}_{2}}\] wan, \[V\] volt are connected in parallel and \[V\] volt supply is applied to them. The total power will be:
A)
\[{{P}_{1}}+{{P}_{2}}\]
done
clear
B)
\[\sqrt{{{P}_{1}}{{P}_{2}}}\]
done
clear
C)
\[\frac{{{P}_{1}}{{P}_{2}}}{{{P}_{1}}+{{P}_{2}}}\]
done
clear
D)
\[\frac{{{P}_{1}}+{{P}_{2}}}{{{P}_{1}}{{P}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 47) A hole is in the bottom of the tank having water. If total pressure at the bottom is \[3\,\,atm\] \[(1\,\,atm={{10}^{5}}N{{m}^{-2}})\], then velocity of water flowing from hole is:
A)
\[\sqrt{400}m{{s}^{-1}}\]
done
clear
B)
\[\sqrt{600}m{{s}^{-1}}\]
done
clear
C)
\[\sqrt{60}m{{s}^{-1}}\]
done
clear
D)
\[\sqrt{40}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 48) A fluid of volume \[1\,\,L\] is subjected to a pressure change of\[{{10}^{7}}N/{{m}^{2}}\]. As a result its volume changes by \[0.4\,\,c{{m}^{3}}\]. The bulk modulus of the fluid is:
A)
\[2.5\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
B)
\[2.5\times {{10}^{11}}N/{{m}^{2}}\]
done
clear
C)
\[2.5\times {{10}^{9}}N/{{m}^{2}}\]
done
clear
D)
\[2.5\times {{10}^{15}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 49) Stationary waves are so called because in them:
A)
the particles of the medium are not disturbed at all
done
clear
B)
the particles of the medium do not execute \[SHM\]
done
clear
C)
Particles do not correspond flow of energy along the wire
done
clear
D)
the interference effect cannot be observed
done
clear
View Answer play_arrow
question_answer 50) If the degrees of freedom of the molecules of a gas are n, the ratio of its two specific heats\[({{C}_{P}}/{{C}_{V}})\]will be:
A)
\[1+\frac{2}{n}\]
done
clear
B)
\[1-\frac{2}{n}\]
done
clear
C)
\[1+\frac{1}{n}\]
done
clear
D)
\[2-\frac{1}{n}\]
done
clear
View Answer play_arrow
question_answer 51) In a radioactive decay:
A)
\[\alpha \] then \[\beta \] and then \[\gamma \] emitted
done
clear
B)
\[\alpha \] or \[\beta \] and then \[\gamma \] emitted
done
clear
C)
\[\alpha \] and \[\beta \] and \[\gamma \] emitted simultaneously
done
clear
D)
\[\alpha \] and \[\beta \] emitted simultaneously
done
clear
View Answer play_arrow
question_answer 52) The work function of a metal is \[1\,\,eV\]. If \[3000\overset{\text{o}}{\mathop{\text{A}}}\,\] wavelength light is incident, the value of stopping voltage is:
A)
\[1\,\,V\]
done
clear
B)
\[3.75\,\,V\]
done
clear
C)
\[3.2\,\,V\]
done
clear
D)
\[0.75\,\,V\]
done
clear
View Answer play_arrow
question_answer 53) Equation for a real gas is\[\left( P+\frac{a}{{{V}^{2}}} \right)(V-b)=RT\]where P= pressure, \[V=\] volume, \[a,\,\,b\] and \[R\] constants, dimensional formula of \[a\] is:
A)
\[{{L}^{6}}\]
done
clear
B)
\[{{M}^{1}}{{L}^{-1}}{{T}^{2}}\]
done
clear
C)
\[{{M}^{1}}{{L}^{5}}{{T}^{-2}}\]
done
clear
D)
\[{{L}^{3}}\]
done
clear
View Answer play_arrow
question_answer 54) A monoatomic ideal gas is compressed to its \[1/8\] volume adiabatically at \[{{17}^{o}}C\]. Temperature after compression will be:
A)
\[{{34}^{o}}C\]
done
clear
B)
\[{{17}^{o}}C\]
done
clear
C)
\[{{136}^{o}}C\]
done
clear
D)
\[{{887}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 55) Which quantity remains constant in adiabatic process?
A)
Work
done
clear
B)
Heat
done
clear
C)
Temperature
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 56) Which formula is incorrect for root mean square velocity?
A)
\[\sqrt{\frac{3RT}{M}}\]
done
clear
B)
\[\sqrt{\frac{3PV}{M}}\]
done
clear
C)
\[\sqrt{\frac{3d}{P}}\]
done
clear
D)
\[\sqrt{\frac{2KE}{M}}\]
done
clear
View Answer play_arrow
question_answer 57) If the number of molecules of hydrogen is double to that of oxygen, at the same temperature, the ratio of their average \[KE\] per molecule is:
A)
\[1:1\]
done
clear
B)
\[2:3\]
done
clear
C)
\[1:2\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 58) A liquid boils at that temperature at which the pressure of saturated vapour is:
A)
more than atmospheric pressure
done
clear
B)
double to atmospheric pressure
done
clear
C)
equal to atmospheric pressure
done
clear
D)
less than atmospheric pressure
done
clear
View Answer play_arrow
question_answer 59) What is the value of \[\frac{PV}{T}\] for a \[1\] mole of gas?
A)
\[4.2\times {{10}^{7}}cal/K\]
done
clear
B)
\[4.2cal/K\]
done
clear
C)
\[8.31cal/K\]
done
clear
D)
\[2cal/K\]
done
clear
View Answer play_arrow
question_answer 60) If a proton totally converts into energy, the value of energy will be:
A)
\[190\,\,MeV\]
done
clear
B)
\[931\,\,MeV\]
done
clear
C)
\[93.1\,\,MeV\]
done
clear
D)
\[931\,\,J\]
done
clear
View Answer play_arrow
question_answer 61) Experimental verification of matter waves was done by:
A)
de-Broglie
done
clear
B)
Rutherford
done
clear
C)
Bohr
done
clear
D)
Davisson and Germer
done
clear
View Answer play_arrow
question_answer 62) In the following nuclear reaction\[_{2}H{{e}^{3}}{{+}_{z}}{{X}^{a}}{{\xrightarrow{{}}}_{z+1}}{{Y}^{a+2}}+Q\]. What is\[Q\]?
A)
Neutron
done
clear
B)
Proton
done
clear
C)
Positron
done
clear
D)
Electron
done
clear
View Answer play_arrow
question_answer 63) For the reaction\[_{1}{{H}^{2}}{{+}_{1}}{{H}^{2}}{{\xrightarrow{{}}}_{2}}H{{e}^{4}}+v+\]Energy, what is the required condition?
A)
High temperature and pressure
done
clear
B)
High temperature and low pressure
done
clear
C)
Low temperature and high pressure
done
clear
D)
High temperature only
done
clear
View Answer play_arrow
question_answer 64) Work function of photoelectric metal is\[3.13\,\,eV\]. Threshold frequency is:
A)
\[4\times {{10}^{11}}Hz\]
done
clear
B)
\[5\times {{10}^{14}}Hz\]
done
clear
C)
\[8\times {{10}^{15}}Hz\]
done
clear
D)
\[8\times {{10}^{10}}Hz\]
done
clear
View Answer play_arrow
question_answer 65) Nitro group of nitrobenzene is:
A)
\[o-\]directing
done
clear
B)
\[m-\]directing
done
clear
C)
\[p-\]directing
done
clear
D)
\[o,\]and \[p\] directing
done
clear
View Answer play_arrow
question_answer 66) In which compound the number of \[{{3}^{o}}\] carbon is maximum:
A)
2, 5 dimethyl hexane
done
clear
B)
2, 3, 4-trimethyl pentane
done
clear
C)
2, 2, 4, 4-tetramethyl pentane
done
clear
D)
2, 2, 3-trimethyl pentane
done
clear
View Answer play_arrow
question_answer 67) The \[pH\] of a solution of concentration few less than\[1\,\,N\,\,NaOH\]:
A)
between \[13\] and \[14\]
done
clear
B)
between \[12\] and \[13\]
done
clear
C)
between \[0\] and \[1\]
done
clear
D)
between \[1\] and\[2\]
done
clear
View Answer play_arrow
question_answer 68) Dichlorocarbene is:
A)
a neutral divalent species
done
clear
B)
a carbonation
done
clear
C)
a carbanion
done
clear
D)
a free radical
done
clear
View Answer play_arrow
question_answer 69) The geometry of the hybrid orbital, which contains \[20%\] \[s-\]character is:
A)
octahedral
done
clear
B)
tetrahedral
done
clear
C)
trigonal bipyramidal
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 70) Which test is not ideal to distinguish 2-butanol and \[1-\]propanol?
A)
Hydrogenation
done
clear
B)
Iodoform test
done
clear
C)
Lucas test
done
clear
D)
Oxidation test
done
clear
View Answer play_arrow
question_answer 71) In the reaction of \[C{{H}_{2}}=C{{H}_{2}}\] and \[HBr\] initial addition occurs of:
A)
indefinite
done
clear
B)
\[{{H}^{+}}+B{{r}^{-}}\] both at one time
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[B{{r}^{-}}\]
done
clear
View Answer play_arrow
question_answer 72) The hydrocarbon formed by electrolysis of sodium propionate:
A)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) \[0.1\,\,M\,\,C{{H}_{3}}COOH\] is \[1.3%\] ionised. The dissociation constant of it will be:
A)
\[1.69\times {{10}^{-5}}\]
done
clear
B)
\[1.69\times {{10}^{-6}}\]
done
clear
C)
\[1.69\times {{10}^{-4}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 74) Which of the following give dye test?
A)
Aniline
done
clear
B)
Methylamine
done
clear
C)
Diphenylamine
done
clear
D)
Ethylamine
done
clear
View Answer play_arrow
question_answer 75) When ethanamide is heated with \[NaOH\] and \[B{{r}_{2}}\] the compound formed is:
A)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}NC\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 76) The chemical formula of potash alum is \[{{K}_{2}}S{{O}_{4}}\cdot A{{l}_{2}}{{(S{{O}_{4}})}_{3}}x{{H}_{2}}O\], here \[x\] is:
A)
\[7\]
done
clear
B)
\[12\]
done
clear
C)
\[6\]
done
clear
D)
\[24\]
done
clear
View Answer play_arrow
question_answer 77) Brass is an alloy of:
A)
\[Cu+Zn+Fe\]
done
clear
B)
\[Cu+Zn+Ni\]
done
clear
C)
\[Cu+Zn+Sn\]
done
clear
D)
\[Cu+Zn\]
done
clear
View Answer play_arrow
question_answer 78) The electronic configuration of\[F{{e}_{26}}\]is\[[Ar]\]:
A)
\[3{{d}^{8}}4{{s}^{2}}\]
done
clear
B)
\[3{{d}^{7}}4{{s}^{2}}\]
done
clear
C)
\[3{{d}^{6}}4{{s}^{2}}\]
done
clear
D)
\[3{{d}^{5}}4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 79) The rate constant of forward reaction is \[2.38\times {{10}^{-4}}\] and the rate constant of backward reaction is \[4.76\times {{10}^{-5}}\]. The equilibrium constant for the reaction will be:
A)
\[5\]
done
clear
B)
\[5\times {{10}^{-2}}\]
done
clear
C)
\[2\times {{10}^{-4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 80) The element with electronic configuration \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}},\,\,3{{s}^{2}}3{{p}^{6}},\,\,4{{s}^{2}}\] shows same property as:
A)
\[Mo\]
done
clear
B)
\[Rb\]
done
clear
C)
\[Ca\]
done
clear
D)
\[Sr\]
done
clear
View Answer play_arrow
question_answer 81) How much volume of \[0.4\,\,M\,\,NaOH\] is required to neutralise completely \[200\,\,mL\]\[0.5\,\,M\,\,{{H}_{2}}S{{O}_{4}}\] solution?
A)
\[600\,\,mL\]
done
clear
B)
\[300\,\,mL\]
done
clear
C)
\[500\,\,mL\]
done
clear
D)
\[200\,\,mL\]
done
clear
View Answer play_arrow
question_answer 82) The formula of acetaldehyde semi car b a zone:
A)
\[C{{H}_{3}}-CH=NNHCONHC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-CH=N-NHCON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CH=N-NHCON{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-CH=N-NHCONH-CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) One compound reacts with chloroform in presence of \[KOH\] and produces a bad smell (nause odour) compound. The compound formed is:
A)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CN\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}NC\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CN\]
done
clear
View Answer play_arrow
question_answer 84) Phenol, chloroform and caustic potash are heated. The compound formed is:
A)
salicylic acid
done
clear
B)
\[p-\]hydroxy benzaldehyde
done
clear
C)
\[m-\]hydroxy benzaldehyde
done
clear
D)
salicylaldehyde
done
clear
View Answer play_arrow
question_answer 85) \[A+NaOH\xrightarrow{{}}C{{H}_{3}}OH+HCOONa\]is the reaction, compound \[A\] is:
A)
\[HCN\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CN\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 86) The compound obtained by the reaction of acetic anhydride and ammonia is:
A)
\[C{{H}_{3}}COON{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}CN\]
done
clear
C)
\[C{{H}_{3}}CONHC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 87) Metal which does not react with aqueous solution of copper sulphate is:
A)
\[Pb\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 88) When aniline react with acetic anhydride the product formed is:
A)
\[p-\]aminobenzoic acid
done
clear
B)
\[m-\]aminobenzoic acid
done
clear
C)
acetanilide
done
clear
D)
\[o-\]aminobenzoic acid
done
clear
View Answer play_arrow
question_answer 89) \[5SO_{2}^{-}+2MnO_{4}^{-}+6{{H}^{+}}\xrightarrow{{}}5SO_{4}^{2-}\]\[+2M{{n}^{2+}}+2{{H}_{2}}O\]the oxidation number of \[Mn\] changes from:
A)
\[+14\]to\[+4\]
done
clear
B)
\[+6\]to\[+2\]
done
clear
C)
\[-7\]to\[-2\]
done
clear
D)
\[+7\]to\[+2\]
done
clear
View Answer play_arrow
question_answer 90) Which has highest ionisation potential?
A)
\[N\]
done
clear
B)
\[O\]
done
clear
C)
\[{{O}^{+}}\]
done
clear
D)
\[Na\]
done
clear
View Answer play_arrow
question_answer 91) Formic acid and acetic acid may be distinguished by the reaction with:
A)
sodium
done
clear
B)
2, 4-dinitrophenyl hydrazine
done
clear
C)
litmus paper
done
clear
D)
Tollen's reagent
done
clear
View Answer play_arrow
question_answer 92) Maximum melting point is of:
A)
\[MgC{{l}_{2}}\]
done
clear
B)
\[BaC{{l}_{2}}\]
done
clear
C)
\[CaC{{l}_{2}}\]
done
clear
D)
\[BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 93) The process requiring the absorption of energy is:
A)
\[F\xrightarrow{{}}{{F}^{-}}\]
done
clear
B)
\[Cl-C{{l}^{-}}\]
done
clear
C)
\[O\xrightarrow{{}}{{O}^{2-}}\]
done
clear
D)
\[H-{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 94) The number of coordinate bond in a molecule of\[{{H}_{2}}S{{O}_{4}}\]:
A)
\[4\]
done
clear
B)
\[3\]
done
clear
C)
\[2\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 95) Carmelite is:
A)
\[KCl\]
done
clear
B)
\[Sn{{l}_{4}}\]
done
clear
C)
\[Ge{{l}_{4}}\]
done
clear
D)
\[Pb{{I}_{4}}\]
done
clear
View Answer play_arrow
question_answer 96) A piece of magnesium ribbon was heated to redness in an atmosphere of nitrogen and on cooling water was added. The gas evolved was:
A)
ammonia
done
clear
B)
hydrogen
done
clear
C)
nitrogen
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 97) Which of the following halides is least stable and doubtful existence?
A)
\[C{{l}_{4}}\]
done
clear
B)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[NiS{{O}_{4}}\]
done
clear
D)
\[FeS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 98) Lithopone is a mixture of:
A)
\[ZnS\]and\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[CaC{{l}_{2}}\]and\[C{{a}_{3}}{{F}_{2}}\]
done
clear
C)
\[Ca{{C}_{2}}\]and\[C{{a}_{3}}{{N}_{2}}\]
done
clear
D)
\[ZnS+BaS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 99) Iodine is liberated from \[KI\] solution when treated with:
A)
\[ZnS{{O}_{4}}\]
done
clear
B)
\[CuS{{O}_{4}}\]
done
clear
C)
\[NiS{{O}_{4}}\]
done
clear
D)
\[FeS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 100) Chlorine acts as a bleaching agent only in the presence of:
A)
dry air
done
clear
B)
sun light
done
clear
C)
moisture
done
clear
D)
pure oxygen
done
clear
View Answer play_arrow
question_answer 101) If\[\sin x+{{\sin }^{2}}x=1\], then the value of\[{{\cos }^{12}}x+3{{\cos }^{10}}x+3{{\cos }^{8}}x+{{\cos }^{6}}x\]is equal to:
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[-1\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 102) The equations\[\frac{2{{\cos }^{2}}x}{2{{\sin }^{2}}x}=\frac{{{x}^{2}}+1}{{{x}^{2}}},\,\,0\le x\le \frac{x}{2}\]has:
A)
one real solution
done
clear
B)
no real solution
done
clear
C)
more than one real solution
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 103) If\[f(x)=\frac{2x+1}{3x-2}\],then\[fof(2)\]is equal to:
A)
\[1\]
done
clear
B)
\[3\]
done
clear
C)
\[4\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 104) Which one of the following objective function on the set of real numbers?
A)
\[2x-5\]
done
clear
B)
\[|x|\]
done
clear
C)
\[{{x}^{2}}\]
done
clear
D)
\[{{x}^{2}}+1\]
done
clear
View Answer play_arrow
question_answer 105) Let the function \[f\]be defined by\[f(x)=\frac{2x+1}{1-3x},\] then\[{{f}^{-1}}(x)\]is:
A)
\[\frac{x-1}{3x+2}\]
done
clear
B)
\[\frac{3x+2}{x-1}\]
done
clear
C)
\[\frac{x+1}{3x-2}\]
done
clear
D)
\[\frac{2x+1}{1-3x}\]
done
clear
View Answer play_arrow
question_answer 106) If\[\sqrt{a+ib}=x+iy\], then possible value of\[\sqrt{a-ib}\]is:
A)
\[{{x}^{2}}+{{y}^{2}}\]
done
clear
B)
\[\sqrt{{{x}^{2}}-{{y}^{2}}}\]
done
clear
C)
\[x+iy\]
done
clear
D)
\[x-iy\]
done
clear
View Answer play_arrow
question_answer 107) If\[{{i}^{2}}=-1\]. Then sum\[i+{{i}^{2}}+{{i}^{3}}+....+{{i}^{1000}}\] term is equal to:
A)
\[1\]
done
clear
B)
\[-1\]
done
clear
C)
\[i\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 108) If\[\alpha ,\,\,\beta \]are the roots of the equation\[{{x}^{2}}+2x+4=0\], then\[\frac{1}{{{\alpha }^{3}}}+\frac{1}{{{\beta }^{3}}}\]is equal to:
A)
\[-1/2\]
done
clear
B)
\[1/4\]
done
clear
C)
\[{{3}^{2}}\]
done
clear
D)
\[1/{{3}^{2}}\]
done
clear
View Answer play_arrow
question_answer 109) The least integer \[k\] which makes roots of the equation \[{{x}^{2}}+5x+k=0\] become imaginary, is:
A)
\[4\]
done
clear
B)
\[5\]
done
clear
C)
\[6\]
done
clear
D)
\[\frac{25}{4}\]
done
clear
View Answer play_arrow
question_answer 110) The number of terms of the \[AP\,\,3,\,\,7,\,\,11,\,\,15...\] to be taken so that the sum is\[210,\,\,\,\text{is}:\]
A)
\[10\]
done
clear
B)
\[12\]
done
clear
C)
-1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 111) If \[a,\,\,b,\,\,c,\] are respectively the \[pth,\,\,qth,\,\,rth\] terms of an\[AP\], then \[\left[ \begin{matrix} a & p & 1 \\ b & q & 1 \\ c & r & 1 \\ \end{matrix} \right]\] is equal to:
A)
\[1\]
done
clear
B)
\[-1\]
done
clear
C)
\[0\]
done
clear
D)
\[pqr\]
done
clear
View Answer play_arrow
question_answer 112) The two geometric means between the numbers \[1\] and \[64\] are:
A)
\[1\] and \[64\]
done
clear
B)
\[4\] and \[16\]
done
clear
C)
\[2\] and\[16\]
done
clear
D)
\[8\] and \[16\]
done
clear
View Answer play_arrow
question_answer 113) If \[n\] and \[r\] are two positive integers such that\[n\ge r\], then\[^{n}{{C}_{r-1}}{{+}^{n}}{{C}_{r}}\]is equal to:
A)
\[^{n}{{C}_{r-1}}\]
done
clear
B)
\[^{n}{{C}_{r}}\]
done
clear
C)
\[^{n-1}{{C}_{r}}\]
done
clear
D)
\[^{n+1}{{C}_{r}}\]
done
clear
View Answer play_arrow
question_answer 114) The number of ways in which a committee of a \[6\] members can be formed from \[8\] gentlemen and \[4\] ladies so that the committee contains atleast \[3\] ladies, is:
A)
\[252\]
done
clear
B)
\[672\]
done
clear
C)
\[420\]
done
clear
D)
\[250\]
done
clear
View Answer play_arrow
question_answer 115) If \[A\] and \[B\] are \[2\] square matrices of the same order, then\[{{(A-B)}^{2}}\]is:
A)
\[{{A}^{2}}-AB-BA+{{B}^{2}}\]
done
clear
B)
\[{{A}^{2}}-2BA+{{B}^{2}}\]
done
clear
C)
\[{{A}^{2}}-2AB+{{B}^{2}}\]
done
clear
D)
\[{{A}^{2}}-{{B}^{2}}\]
done
clear
View Answer play_arrow
question_answer 116) If \[I\] is a unit matrix of order \[10\], then determinant of \[I\] is equal to:
A)
\[10\]
done
clear
B)
\[1\]
done
clear
C)
\[1/10\]
done
clear
D)
\[9\]
done
clear
View Answer play_arrow
question_answer 117) If the vectors \[3\widehat{\mathbf{i}}+\lambda \widehat{\mathbf{j}}+\widehat{\mathbf{k}}\] and \[2\widehat{\mathbf{i}}-\widehat{\mathbf{j}}+8\widehat{\mathbf{k}}\] are perpendicular, then value of \[\lambda \] is:
A)
\[-14\]
done
clear
B)
\[7\]
done
clear
C)
\[14\]
done
clear
D)
\[1/7\]
done
clear
View Answer play_arrow
question_answer 118) The unit vector perpendicular to both \[\widehat{\mathbf{i}}-\widehat{\mathbf{j}}\] and \[\widehat{\mathbf{j}}+\widehat{\mathbf{k}}\] is:
A)
\[\widehat{\mathbf{i}}-\widehat{\mathbf{j}}+\widehat{\mathbf{k}}\]
done
clear
B)
\[\widehat{\mathbf{i}}+\widehat{\mathbf{j}}+\widehat{\mathbf{k}}\]
done
clear
C)
\[\frac{\widehat{\mathbf{i}}+\widehat{\mathbf{j}}+\widehat{\mathbf{k}}}{\sqrt{3}}\]
done
clear
D)
\[\frac{\widehat{\mathbf{i}}+\widehat{\mathbf{j}}-\widehat{\mathbf{k}}}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 119) For any three vectors\[\overset{\to }{\mathop{\mathbf{a}}}\,,\,\,\overset{\to }{\mathop{\mathbf{b}}}\,,\,\,\overset{\to }{\mathop{\mathbf{c}}}\,\];\[\overset{\to }{\mathop{\mathbf{a}}}\,\times (\overset{\to }{\mathop{\mathbf{b}}}\,+\overset{\to }{\mathop{\mathbf{c}}}\,)+\overset{\to }{\mathop{\mathbf{b}}}\,\times (\overset{\to }{\mathop{\mathbf{c}}}\,+\overset{\to }{\mathop{\mathbf{a}}}\,)+\overset{\to }{\mathop{\mathbf{c}}}\,\times (\overset{\to }{\mathop{\mathbf{a}}}\,+\overset{\to }{\mathop{\mathbf{b}}}\,)\]is:
A)
\[\overset{\to }{\mathop{\mathbf{0}}}\,\]
done
clear
B)
\[\overset{\to }{\mathop{\mathbf{a}}}\,+\overset{\to }{\mathop{\mathbf{b}}}\,+\overset{\to }{\mathop{\mathbf{c}}}\,\]
done
clear
C)
\[\overset{\to }{\mathop{\mathbf{a}}}\,\cdot (\overset{\to }{\mathop{\mathbf{b}}}\,\times \overset{\to }{\mathop{\mathbf{c}}}\,)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 120) The point moves such that the area of the triangle formed by it with the points \[(1,\,\,5)\] and \[(3,\,\,-7)\] is\[21\,\,sq\,\,unit\]The locus of the point is:
A)
\[6x+y-32=0\]
done
clear
B)
\[6x-y+32=0\]
done
clear
C)
\[x+6y-32=0\]
done
clear
D)
\[6x+y+32=0\]
done
clear
View Answer play_arrow
question_answer 121) The line \[\frac{x}{a}-\frac{y}{b}=1\] cuts the \[x-\]axis at \[P\]. The equation of the line through \[P\] perpendicular to the given line is:
A)
\[x+y=ab\]
done
clear
B)
\[x+y=a+b\]
done
clear
C)
\[ax+by={{a}^{2}}\]
done
clear
D)
\[bx+ay={{b}^{2}}\]
done
clear
View Answer play_arrow
question_answer 122) Three vertices of a parallelogram taken in order are \[(-1,\,\,-6),\,\,\,(2,\,\,-5)\] and \[(7,\,\,2)\]. The fourth vertex is:
A)
\[(1,\,\,4)\]
done
clear
B)
\[(4,\,\,1)\]
done
clear
C)
\[(1,\,\,1)\]
done
clear
D)
\[(4,\,\,4)\]
done
clear
View Answer play_arrow
question_answer 123) Distance between the lines \[5x+3y-7=0\] and\[15x+9y+14=0\] is:
A)
\[\frac{35}{\sqrt{34}}\]
done
clear
B)
\[\frac{1}{3\sqrt{34}}\]
done
clear
C)
\[\frac{35}{3\sqrt{34}}\]
done
clear
D)
\[\frac{35}{2\sqrt{34}}\]
done
clear
View Answer play_arrow
question_answer 124) If the equation\[2{{x}^{2}}+7xy+3{{y}^{2}}-9x-7y+k=0\] represents a pair of lines, then \[k\] is equal to:
A)
\[4\]
done
clear
B)
\[2\]
done
clear
C)
\[1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) The centre of a circle \[(2,\,\,-3)\] and the circumference is \[10\pi \]. The equation or the circle is:
A)
\[{{x}^{2}}+{{y}^{2}}+4x+6y+12=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-4x+6y+12=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}-4x+6y-12=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}-4x-6y-12=0\]
done
clear
View Answer play_arrow
question_answer 126) The equation of the parabola whose vertex is at \[(2,\,\,-1)\] and focus at \[(2,\,\,-3)\] is:
A)
\[{{x}^{2}}+4x-8y-12=0\]
done
clear
B)
\[{{x}^{2}}-4x+8y+12=0\]
done
clear
C)
\[{{x}^{2}}+8y=12\]
done
clear
D)
\[{{x}^{2}}-4x+12=0\]
done
clear
View Answer play_arrow
question_answer 127) The eccentricity of the conic \[9{{x}^{2}}+25{{y}^{2}}=225\] is:
A)
\[2/5\]
done
clear
B)
\[4/5\]
done
clear
C)
\[1/3\]
done
clear
D)
\[1/5\]
done
clear
View Answer play_arrow
question_answer 128) If\[{{\tan }^{-1}}x+{{\tan }^{-1}}y+{{\tan }^{-1}}z=\pi \], then\[x+y+z\]is equal to:
A)
\[xyz\]
done
clear
B)
\[0\]
done
clear
C)
\[1\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}+{{z}^{2}}\]
done
clear
View Answer play_arrow
question_answer 129) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{1-\cos mx}{1-\cos nx}\]is:
A)
\[m/n\]
done
clear
B)
\[{{m}^{2}}/{{n}^{2}}\]
done
clear
C)
\[0\]
done
clear
D)
\[n/m\]
done
clear
View Answer play_arrow
question_answer 130) If\[x={{\sin }^{-1}}(3t-4{{t}^{3}})\]and\[y={{\cos }^{-1}}\sqrt{(1-{{t}^{2}})}\], then \[\frac{dy}{dx}\] is equal to:
A)
\[1/2\]
done
clear
B)
\[2/5\]
done
clear
C)
\[3/2\]
done
clear
D)
\[1/3\]
done
clear
View Answer play_arrow
question_answer 131) The second derivative of a sin31 with respect to\[a{{\cos }^{3}}t\]at\[t=\frac{\pi }{4}\]to:
A)
\[\frac{4\sqrt{2}}{3a}\]
done
clear
B)
\[2\]
done
clear
C)
\[\frac{1}{12a}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 132) If\[f(x)=\left\{ \begin{matrix} \frac{{{x}^{2}}-9}{x-3}, & if\,\,x\ne 3 \\ 2x+k, & if\,\,x=3 \\ \end{matrix} \right.\]is continuous at\[x=3\], then \[k\] is equal to:
A)
\[3\]
done
clear
B)
\[0\]
done
clear
C)
\[-6\]
done
clear
D)
\[1/6\]
done
clear
View Answer play_arrow
question_answer 133) The function\[y=a(1-\cos x),\,\,a>0\]is maximum when \[x\] is equal to:
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[-\frac{\pi }{2}\]
done
clear
D)
\[-\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 134) \[\int_{0}^{\pi /2}{x\,\,\sin \,\,x\,\,dx}\]is equal to:
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 135) \[\int_{0}^{\pi /2}{\frac{\sin x}{\sin x+\cos x}}dx\]is equal to:
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{3}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 136) The area bounded by the parabola \[{{y}^{2}}=4ax\] and\[{{x}^{2}}=4ay\]is:
A)
\[\frac{8{{a}^{2}}}{3}sq\,\,unit\]
done
clear
B)
\[\frac{16{{a}^{2}}}{3}sq\,\,unit\]
done
clear
C)
\[\frac{32{{a}^{2}}}{3}sq\,\,unit\]
done
clear
D)
\[\frac{64{{a}^{2}}}{3}sq\,\,unit\]
done
clear
View Answer play_arrow
question_answer 137) The degree of differential equation \[\frac{{{d}^{2}}y}{d{{x}^{2}}}+{{\left( \frac{dy}{dx} \right)}^{3}}+6y=0\]is:
A)
\[1\]
done
clear
B)
\[3\]
done
clear
C)
\[2\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 138) The solution\[\frac{dy}{dx}+P(x)y=0\]is:
A)
\[y=c{{e}^{\int{P\,\,dx}}}\]
done
clear
B)
\[x=c{{e}^{-\int{P\,\,dx}}}\]
done
clear
C)
\[y=c{{e}^{-\int{P\,\,dx}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 139) If\[P(A)=2/3,\,\,P(B)=1/2\]and\[P(A\cup B)=5/6\], then events \[A\] and \[B\] are:
A)
mutually, exclusive
done
clear
B)
independent as well as mutually exhaustive
done
clear
C)
independent
done
clear
D)
dependent only on\[A\]
done
clear
View Answer play_arrow
question_answer 140) \[\int_{-2}^{2}{|1-{{x}^{2}}|dx}\]is equal to:
A)
\[4\]
done
clear
B)
\[2\]
done
clear
C)
\[-2\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 141) \[\int_{2}^{4}{x}\sqrt{6-x}\,dx\]is equal to:
A)
\[\frac{16}{5}(3-\sqrt{2})\]
done
clear
B)
\[\frac{32}{5}(3-\sqrt{2})\]
done
clear
C)
\[\frac{8}{5}(3-\sqrt{2})\]
done
clear
D)
\[\frac{64}{5}(3-\sqrt{2})\]
done
clear
View Answer play_arrow
question_answer 142) \[\int_{a}^{b}{\frac{f(x)}{f(x)+f(a+b-x)}}dx\]is equal to:
A)
\[\frac{b-a}{2}\]
done
clear
B)
\[\frac{a-b}{2}\]
done
clear
C)
\[\frac{a}{2}\]
done
clear
D)
\[\frac{b}{2}\]
done
clear
View Answer play_arrow
question_answer 143) The maximum value of the function\[\sin x(1+\cos x)\]is:
A)
\[3\]
done
clear
B)
\[\frac{3\sqrt{3}}{4}\]
done
clear
C)
\[4\]
done
clear
D)
\[3\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 144) Let\[f(x)=\frac{1}{\sqrt{18-{{x}^{2}}}}\]. The value of\[\underset{x\to 3}{\mathop{\lim }}\,\frac{f(x)-f(3)}{x-3}\]is:
A)
\[0\]
done
clear
B)
\[-1/9\]
done
clear
C)
\[-1/3\]
done
clear
D)
\[3\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 145) \[{{x}^{y}}\cdot {{y}^{x}}=1\], then\[\frac{dy}{dx}\]is:
A)
\[\frac{y(y+x\log y)}{x(y\log x+x)}\]
done
clear
B)
\[\frac{y(x+y\log x)}{x(y+x\log y)}\]
done
clear
C)
\[\frac{-y(y+x\log y)}{x(x+y\log x)}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 146) If in \[\Delta ABC\], \[a+\tan A+b\tan B\]\[=(a+b)\tan \frac{1}{2}(A+B)\], then:
A)
\[A=B\]
done
clear
B)
\[B=C\]
done
clear
C)
\[C=A\]
done
clear
D)
\[A=B=C\]
done
clear
View Answer play_arrow
question_answer 147) The centre of the ellipse\[\frac{{{(x+y-2)}^{2}}}{9}+\frac{{{(x-y)}^{2}}}{16}=1\]is:
A)
\[(0,\,\,0)\]
done
clear
B)
\[(1,\,\,1)\]
done
clear
C)
\[(1,\,\,0)\]
done
clear
D)
\[(0,\,\,1)\]
done
clear
View Answer play_arrow
question_answer 148) The angle between the pair of lines is given by the equation\[{{x}^{2}}+2xy-{{y}^{2}}=0\]is:
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{\pi }{6}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 149) The term independent of\[x\]is\[{{\left( \frac{3}{2}{{x}^{2}}-\frac{1}{3x} \right)}^{9}}\], is:
A)
\[5/27\]
done
clear
B)
\[7/18\]
done
clear
C)
\[8/27\]
done
clear
D)
\[1/24\]
done
clear
View Answer play_arrow
question_answer 150) If\[x\]is real, then\[\frac{{{x}^{2}}-2x+4}{{{x}^{2}}+2x+4}\]takes values in the interval:
A)
\[[1/3,\,\,3]\]
done
clear
B)
\[(1/3,\,\,3)\]
done
clear
C)
\[(3,\,\,3)\]
done
clear
D)
\[(-1/3,\,\,3)\]
done
clear
View Answer play_arrow