A) \[C{{H}_{4}}<{{H}_{2}}O<N{{H}_{3}}\]
B) \[{{H}_{2}}O<N{{H}_{3}}<C{{H}_{4}}\]
C) \[N{{H}_{3}}<C{{H}_{4}}<{{H}_{2}}O\]
D) \[N{{H}_{3}}<{{H}_{2}}O<C{{H}_{4}}\]
Correct Answer: B
Solution :
Bond angles depend upon number of lone pair (s) of electrons. Higher the number of lone pairs of electrons, lesser the bond angle.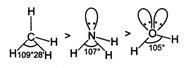
You need to login to perform this action.
You will be redirected in
3 sec
