A) \[s{{p}^{3}}\] only
B) \[s{{p}^{2}}\]and \[s{{p}^{3}}\]
C) \[s{{p}^{2}}\]only
D) sp only
Correct Answer: A
Solution :
When ethanol vapours are passed over alumina at \[\text{260}{{\,}^{\text{o}}}\text{C}\]it undergoes partial dehydration and gives ether while at higher temperature (ie, \[\text{360}{{\,}^{\text{o}}}\text{C}\]), it gives ethene. \[\underset{\text{vapours}}{\mathop{{{\text{C}}_{2}}{{H}_{5}}OH}}\,\xrightarrow[260{{\,}^{o}}C]{A{{l}_{2}}{{O}_{3}}}\underset{\begin{smallmatrix} (X) \\ ether \end{smallmatrix}}{\mathop{{{C}_{2}}{{H}_{5}}-O{{C}_{2}}{{H}_{5}}}}\,\] The structure of X can be written as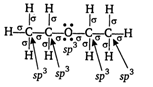 Hence, in product X all the atoms are \[\text{s}{{\text{p}}^{\text{3}}}\]hybridised.
Hence, in product X all the atoms are \[\text{s}{{\text{p}}^{\text{3}}}\]hybridised.
You need to login to perform this action.
You will be redirected in
3 sec
