A)
\[X\] \[Y\] \[s{{p}^{2}},\] angular \[s{{p}^{3}},\] tetrahedral
B)
\[X\] \[Y\] \[s{{p}^{2}},\] angular \[s{{p}^{2}},\] angular
C)
\[X\] \[Y\] \[s{{p}^{2}},\] angular \[s{{p}^{2}},\] planar triangular
D)
\[X\] \[Y\] \[s{{p}^{3}},\]planar \[s{{p}^{3}},\]planar
Correct Answer: C
Solution :
\[\underset{\text{sulphurous}\,\text{acid}}{\mathop{{{H}_{2}}S{{O}_{3}}}}\,\xrightarrow[-2{{H}_{2}}O]{{{P}_{2}}{{O}_{5}}}\underset{\begin{smallmatrix} \,\,\,\,\,\,\,\,\,\,\,\,\,(X) \\ (anydride\,of\, \\ sulphurous\,acid) \end{smallmatrix}}{\mathop{S{{O}_{2}}}}\,\]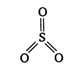 \[\Rightarrow \] \[3bp+01p\] Thus, in \[S{{O}_{3}}\]hybridisation of S is \[s{{p}^{2}}\]and its shape is triangular planar.
\[\Rightarrow \] \[3bp+01p\] Thus, in \[S{{O}_{3}}\]hybridisation of S is \[s{{p}^{2}}\]and its shape is triangular planar.
You need to login to perform this action.
You will be redirected in
3 sec
