A) \[X=4,Y={{90}^{o}}\]
B) \[X=4,\text{ }Y={{60}^{o}}\]
C) \[X=3,\text{ }Y={{120}^{o}}\]
D) \[X=2,Y={{180}^{o}}\]
Correct Answer: B
Solution :
White phosphorus has the molecular formula \[{{\text{P}}_{4}}\]both in solid and vapour state at moderate temperature. The four atoms present in the molecule are arranged at the comers of a tetrahedro so, the PPP bond angle is \[\text{6}{{\text{0}}^{\text{o}}}.\] At higher temperature (above \[\text{700}{{\,}^{\text{o}}}\text{C}\]), it dissociates to give diatomic molecules as: \[{{P}_{4}}\rightleftharpoons 2{{P}_{2}}\] Structure of \[{{\text{P}}_{\text{4}}}\]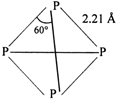
You need to login to perform this action.
You will be redirected in
3 sec
