A) methyl ethanoate
B) methyl methanoate
C) ethyl methanoate
D) methyl propanoate
Correct Answer: A
Solution :
Hydrolysis of \[\text{C}{{\text{H}}_{\text{3}}}\text{COOC}{{\text{H}}_{\text{3}}}\] (methyl ethanoate) gives acetic acid and methyl alcohol. Acetic acid on electrolysis by Kolbes method gives ethane. So, the ester is methyl ethanoate. \[\underset{\text{Methyl}\,\text{ethanoate}}{\mathop{C{{H}_{3}}CO}}\,\,OH\xrightarrow{{}}\] \[\underset{\text{Acetic}\,\text{acid}}{\mathop{C{{H}_{3}}COOH+}}\,C{{H}_{3}}OH\] Kolbes electrolysis: \[\underset{\text{Acetic}\,\text{acid}}{\mathop{C{{H}_{3}}COOH}}\,\xrightarrow{{}}C{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\] At anode: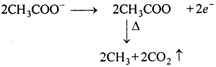 or\[\underset{(Ethane)}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\] At cathode: \[{{H}^{+}}+{{e}^{-}}\xrightarrow{{}}H\] \[H+H\xrightarrow{{}}{{H}_{2}}\uparrow \]
or\[\underset{(Ethane)}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\] At cathode: \[{{H}^{+}}+{{e}^{-}}\xrightarrow{{}}H\] \[H+H\xrightarrow{{}}{{H}_{2}}\uparrow \]
You need to login to perform this action.
You will be redirected in
3 sec
