A) \[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
B) \[{{[Co{{(NH)}_{3}})}_{6}}{{]}^{3+}}\]
C) \[{{[CoCl]}_{6}}{{]}^{3-}}\]
D) \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
Correct Answer: C
Solution :
[a] In \[{{[Cr{{(CN)}_{6}}]}^{3-}}\] and \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}\] both, Cr is present as \[C{{r}^{3+}}\].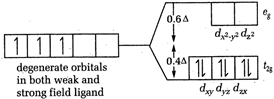 \[CFSE=-0.4\times 3=-1.2\] [b] In \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\], Co is present as \[C{{o}^{3+}}\]
\[CFSE=-0.4\times 3=-1.2\] [b] In \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\], Co is present as \[C{{o}^{3+}}\] 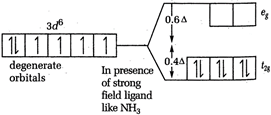 \[CFSE=-0.4\times 6\]= - 2.4 [c] In \[{{[CoC{{l}_{6}}]}^{3-}}Co\], is again present as \[C{{o}^{2+}}\]
\[CFSE=-0.4\times 6\]= - 2.4 [c] In \[{{[CoC{{l}_{6}}]}^{3-}}Co\], is again present as \[C{{o}^{2+}}\] 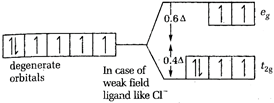 \[CFSE=-0.4\times 4+0.6\times 2=-1.6+1.2\] = - 0.4 Thus, the magnitude of \[{{\Delta }_{o}}\] is minimum for \[{{[CoC{{l}_{6}}]}^{3-}}\]-.
\[CFSE=-0.4\times 4+0.6\times 2=-1.6+1.2\] = - 0.4 Thus, the magnitude of \[{{\Delta }_{o}}\] is minimum for \[{{[CoC{{l}_{6}}]}^{3-}}\]-.
You need to login to perform this action.
You will be redirected in
3 sec
