A) 0
B) 2
C) 3
D) 4
Correct Answer: A
Solution :
In \[[Ni{{(CO)}_{4}}]\], Ni is present as Ni atom. \[Ni=[Ar]\,3{{d}^{8}}4{{s}^{2}}\] CO, being a strong field ligand, transfers the 4s-electrons into 3d-orbitals. \[[Ni{{(CO)}_{4}}]=[Ar]\]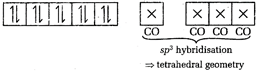 Thus, \[[Ni{{(CO)}_{4}}]\]does not contain any unpaired electron.
Thus, \[[Ni{{(CO)}_{4}}]\]does not contain any unpaired electron.
You need to login to perform this action.
You will be redirected in
3 sec
