A) Only [a]
B) Only [b]
C) Only [c]
D) Both [b] and [c]
Correct Answer: B
Solution :
\[\underset{a}{\mathop{C{{H}_{3}}}}\,-\underset{b}{\mathop{C}}\,{{H}_{2}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\underset{c}{\mathop{C{{H}_{3}}}}\,\] This compound undergoes embolization by the following mechanism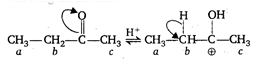 \[\xrightarrow[-{{H}^{+}}]{{{H}_{2}}O}C{{H}_{3}}-CH=\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] Thus, hydrogen [b] is most acidic as it is attached with the active methylene group.
\[\xrightarrow[-{{H}^{+}}]{{{H}_{2}}O}C{{H}_{3}}-CH=\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] Thus, hydrogen [b] is most acidic as it is attached with the active methylene group.
You need to login to perform this action.
You will be redirected in
3 sec
