A) \[Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
B) \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{3}}\]
C) \[Cl-CH=CH-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
D) \[H-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,=C=CH-C{{H}_{2}}-C{{H}_{3}}\]
Correct Answer: D
Solution :
A molecule is said to be chiral or asymmetric, if it does not possess any element of symmetry and not superimposable on its mirror image and this property of die molecule to show non-superimposability is called chirality. [a] \[ClC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\] achiral molecule, absence of asymmetrical carbon atom. [b] \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-CH-C{{H}_{3}}\] achiral molecule, absence of assymmetrical carbon atom. [c] \[\underset{achiral\text{ }molecule}{\mathop{Cl-CH=CH-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}}}\,\] [d]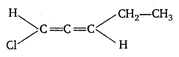 Allenes exhibits optical isomerism Central), provided that the two groups attached to each terminal carbon atoms are different.
Allenes exhibits optical isomerism Central), provided that the two groups attached to each terminal carbon atoms are different.
You need to login to perform this action.
You will be redirected in
3 sec
