A) Cannizaros reaction
B) Perkins reaction
C) Tischenko reaction
D) Reimer-Tiemann reaction
Correct Answer: D
Solution :
[a] Cannizaro reaction: During this reaction aldehydes which do not have a-hydrogen under disproportionation reaction in presence of aq. alkali half of the molecules are oxidised and other half are reduced. \[\underset{formaldehyde}{\mathop{HCHO}}\,+NaOH\to \underset{sodium\text{ }formate}{\mathop{HCOONa}}\,\]\[+\,\,\underset{methyl\text{ }alcohol}{\mathop{C{{H}_{3}}OH}}\,\] [b] Perkin condensation: When aromatic aldehydes react with aliphatic acid anhydride in presence of sodium salt of the same acid as base, they form \[\alpha ,\,\beta \]-unsaturated acid. \[\underset{benzaldehyde}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,\,\,+\underset{acetic\text{ }anhydride}{\mathop{{{(C{{H}_{3}}CO)}_{2}}O}}\,\xrightarrow{C{{H}_{3}}COONa}\]\[\underset{cinnamic\text{ }acid}{\mathop{{{C}_{6}}{{H}_{5}}CH=CHCOOH}}\,+C{{H}_{3}}COOH\] [c] Tischenko reaction: All the aldehydes on reaction with aluminium ethoxide form esters. \[\underset{propanaldehyde}{\mathop{2C{{H}_{3}}C{{H}_{2}}CHO}}\,\xrightarrow{Al{{[O{{C}_{2}}{{H}_{5}})}_{3}}}\]\[\underset{n-propylpropionate}{\mathop{C{{H}_{3}}C{{H}_{2}}COO{{C}_{3}}{{H}_{7}}}}\,\]\[+{{H}_{2}}O\] [d] Reimer-Tiemann reaction: Phenol reacts with CCl4 in presence of \[NaOH\] at 340 K to form salicylic acid.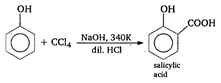
You need to login to perform this action.
You will be redirected in
3 sec
