question_answer 1) Let a body starts from rest with uniform acceleration and after n sec its velocity is v, its displacement in the last two seconds will be
A)
\[\frac{2v\,(n-1)}{n}\]
done
clear
B)
\[\frac{v\,(n-1)}{n}\]
done
clear
C)
\[\frac{2v\,(n+1)}{n}\]
done
clear
D)
\[\frac{v\,(n+1)}{n}\]
done
clear
View Answer play_arrow
question_answer 2) A car A travelling on a straight road with uniform speed of 60\[km{{h}^{-1}}\] is followed by another car B moving at a speed of 70\[km{{h}^{-1}}\]. If the distance between the two cars is 2.5 km and the car B starts deceleration at the rate of 20\[km{{h}^{-1}}\], then after how much time will B catches A?
A)
\[\frac{1}{8}h\]
done
clear
B)
\[\frac{1}{4}h\]
done
clear
C)
\[\frac{1}{2}h\]
done
clear
D)
\[1\,h\]
done
clear
View Answer play_arrow
question_answer 3) If \[{{\mu }_{0}}\] and \[{{\varepsilon }_{0}}\] denote the permeability and permittivity of free space, then the dimensions of \[{{\mu }_{0}}{{\varepsilon }_{0}}\] are
A)
\[[{{M}^{0}}{{L}^{-2}}{{T}^{2}}]\]
done
clear
B)
\[[L{{T}^{-1}}]\]
done
clear
C)
\[[{{M}^{-1}}{{L}^{-3}}{{Q}^{2}}{{T}^{2}}]\]
done
clear
D)
\[[{{M}^{-2}}{{L}^{-1}}{{I}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 4) If two masses of 2 kg and 3 kg are attached to the end of the string passed over a pulley fixed at the top, then the tension and the acceleration are
A)
\[\frac{21}{8}g,\,\frac{g}{8}\]
done
clear
B)
\[\frac{12g}{5},\,\frac{g}{5}\]
done
clear
C)
\[\frac{21g}{8},\,\frac{g}{5}\]
done
clear
D)
\[\frac{7g}{8},\,\frac{g}{8}\]
done
clear
View Answer play_arrow
question_answer 5) When a motor cyclist moves in a circular track of radius 100 m , then the maximum velocity with which the cyclist can take the turn with leaning inwards is (Take coefficient of friction\[=0.2\,T\])
A)
\[1.4\,m{{s}^{-1}}\]
done
clear
B)
\[14\,m{{s}^{-1}}\]
done
clear
C)
\[140\,m{{s}^{-1}}\]
done
clear
D)
\[280\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 6) If three resistors each of 40, are connected together to form a network, then the equivalent resistance of the network cannot be
A)
\[3\,\Omega \]
done
clear
B)
\[6\,\Omega \]
done
clear
C)
\[12\,\Omega \]
done
clear
D)
\[1.33\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 7) A winding wire which is used to frame a solenoid can bear a maximum 10Acurrent. If length of solenoid is 80 cm and its cross-sectional radius is 3 cm, then required length of winding wire is (Magnetic field due to solenoid = 0.2 T)
A)
\[1.2\times {{10}^{2}}\,m\]
done
clear
B)
\[2.4\times {{10}^{3}}\,m\]
done
clear
C)
\[4.8\times {{10}^{2}}\,m\]
done
clear
D)
\[6\times {{10}^{3}}\,m\]
done
clear
View Answer play_arrow
question_answer 8) Which of the following statement is true about X-rays and gamma rays?
A)
Gamma rays have smaller frequency than that of X-rays
done
clear
B)
X-rays have smaller wavelength than that of gamma rays
done
clear
C)
X-rays have larger wavelength than that of gamma rays
done
clear
D)
Wavelength and frequency of X-rays are larger than that of gamma rays
done
clear
View Answer play_arrow
question_answer 9) The work function of a metal is\[1.6\times {{10}^{-19}}J,\]if a light of wavelength 6400\[\overset{\text{o}}{\mathop{\text{A}}}\,\] falls on its surface, then the maximum kinetic energy of emitted photo electron will be (Take Plancks constant\[h=6.4\times {{10}^{-34}}J\text{-}s\])
A)
\[1.4\times {{10}^{-19}}eV\]
done
clear
B)
\[1.4\times {{10}^{-19}}J\]
done
clear
C)
\[14\times {{10}^{-19}}J\]
done
clear
D)
\[2.8\times {{10}^{-19}}J\]
done
clear
View Answer play_arrow
question_answer 10) If an electron jumps from orbit n = 4 to the orbit n = 2 in an atom, then the wavelength of the emitted radiation is (Take R = Rydbergs constant)
A)
\[\frac{16}{9R}\]
done
clear
B)
\[\frac{16}{7R}\]
done
clear
C)
\[\frac{16}{5R}\]
done
clear
D)
\[\frac{16}{3R}\]
done
clear
View Answer play_arrow
question_answer 11) In case of a bcc lattice, the nearest distance between two atoms is equal to
A)
\[\frac{a}{\sqrt{2}}\]
done
clear
B)
\[\frac{a\sqrt{2}}{3}\]
done
clear
C)
\[\frac{a\sqrt{3}}{2}\]
done
clear
D)
\[a\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 12) The correct relation between the linear magnification m, the object distance u and the focal length\[f,\] for a mirror is given by
A)
\[m=\frac{f}{f-u}\]
done
clear
B)
\[m=\frac{f-u}{f}\]
done
clear
C)
\[m=\frac{f}{f+u}\]
done
clear
D)
\[m=\frac{f+u}{f}\]
done
clear
View Answer play_arrow
question_answer 13) The direction of magnetic lines of force inside a magnet are
A)
from JV-pole to S-pole of the magnet
done
clear
B)
from S-pole to N-pole of the magnet
done
clear
C)
depends upon the area of cross-section of the bar magnet
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14) When two bulbs connected in parallel, they together consume 48 W from a battery of 6 V. What is the value of resistance each bulb?
A)
6.5\[\Omega \]
done
clear
B)
5.5\[\Omega \]
done
clear
C)
3.5\[\Omega \]
done
clear
D)
1.5\[\Omega \]
done
clear
View Answer play_arrow
question_answer 15) A cylindrical metal wire of length \[l\] and cross-sectional area S, has resistance R, conductance G, conductivity \[\sigma \] and resistivity\[\rho \]. Which one of the following expression for\[\sigma \]is valid?
A)
\[\frac{GS}{l}\]
done
clear
B)
\[\frac{Rl}{S}\]
done
clear
C)
\[\frac{GR}{\rho }\]
done
clear
D)
\[\frac{\rho R}{G}\]
done
clear
View Answer play_arrow
question_answer 16) A capacitor of capacitance 500\[\mu F\] is charged at a steady rate of 100\[\mu C\,{{s}^{-1}}\]. The potential difference across the capacitor will be 10V in time
A)
25 s
done
clear
B)
50 s
done
clear
C)
75 s
done
clear
D)
100 s
done
clear
View Answer play_arrow
question_answer 17) If for a particle of mass 10 g executing SHM along a straight line, the time period is 2 s and amplitude is 10 cm then what will be its kinetic energy when it is at 5 cm from its equilibrium position?
A)
\[0.375\,{{\pi }^{2}}\,\text{erg}\]
done
clear
B)
\[3.75\,{{\pi }^{2}}\,\text{erg}\]
done
clear
C)
\[37.5\,{{\pi }^{2}}\,\text{erg}\]
done
clear
D)
\[375\,{{\pi }^{2}}\,\text{erg}\]
done
clear
View Answer play_arrow
question_answer 18) The mean free path of gas molecules depends on
A)
\[{{d}^{-2}}\]
done
clear
B)
\[{{d}^{2}}\]
done
clear
C)
\[{{d}^{-1}}\]
done
clear
D)
\[d\]
done
clear
View Answer play_arrow
question_answer 19) If suddenly the force of earths gravity disappears then,
A)
mass and weight will remain the same
done
clear
B)
weight of body will become zero but mass remains the same
done
clear
C)
mass of the body become zero but the weight remains the same
done
clear
D)
both the mass and weight will be the same
done
clear
View Answer play_arrow
question_answer 20) A mass of 10 kg connected at the end of a rod of negligible mass is rotating in a circle of radius 30 cm with an angular velocity of 10 s\[\text{rad}{{\text{s}}^{-1}}\]. If the mass is brought to rest in 10 s by a brake, what is the magnitude of the torque applied?
A)
0.5 N-m
done
clear
B)
0.9 N-m
done
clear
C)
1.2N-m
done
clear
D)
2.3 N-m
done
clear
View Answer play_arrow
question_answer 21) Which one of the following statement about satellites is incorrect?
A)
Geostationary satellites are launched in the equatorial plane
done
clear
B)
A satellite cannot move in a stable orbit in a plane passing through earths centre
done
clear
C)
The speed of a satellite increases with an increase in the radius of its orbit
done
clear
D)
All are incorrect
done
clear
View Answer play_arrow
question_answer 22) An ideal gas is expanded adiabatically. How many times has the gas to be expanded to reduce the root mean square velocity of molecules two times? (y= 1.5)
A)
2 times
done
clear
B)
4 times
done
clear
C)
8 times
done
clear
D)
16 times
done
clear
View Answer play_arrow
question_answer 23) The two opposite faces of a cubical piece of iron are at \[100{}^\circ C\] and \[0{}^\circ C\] in ice. If the area of surface is 4\[c{{m}^{2}},\] then the mass of ice melted in 10 min will be (Thermal conductivity of iron = 0.2 CGS units)
A)
5 g
done
clear
B)
30 g
done
clear
C)
50 g
done
clear
D)
300 g
done
clear
View Answer play_arrow
question_answer 24) If two charges +q and -q are placed at certain distance apart, then at the point exactly midway between them
A)
electric field is not zero but potential is zero
done
clear
B)
electric field and potential both are zero.
done
clear
C)
electric field is zero but potential is not zero
done
clear
D)
neither electric field nor potential is zero
done
clear
View Answer play_arrow
question_answer 25) In Youngs double slit experiment, how many maxima can be obtained on a screen (with central maxima) on both sides of the central fringe if d = 7000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] and \[\overset{\text{o}}{\mathop{\text{A}}}\,\]= 2000\[\overset{\text{o}}{\mathop{\text{A}}}\,\]
A)
7
done
clear
B)
10
done
clear
C)
12
done
clear
D)
18
done
clear
View Answer play_arrow
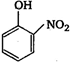
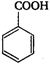
question_answer 26) \[Z\xrightarrow{PC{{l}_{5}}}X\xrightarrow{Alc.\,\,KOH}Y\xrightarrow[2\,{{H}_{2}}O/\Delta ]{Conc.\,\,{{H}_{2}}S{{O}_{4}}}Z\]Here, Z is
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}-C-OH\]
done
clear
C)
\[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 27) Which one of the following statement is incorrect for the sucrose?
A)
It is obtained from cane sugar
done
clear
B)
It is not reducing sugar
done
clear
C)
On hydrolysis, it gives equal quantities of D-glucose and D-fructose
done
clear
D)
It gives aspartame when it is heated at \[210{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 28) Which ligand is useful for the removal of toxic effect of lead metal in body in chelate therapy treatment?
A)
\[\underset{CO{{O}^{-}}}{\overset{CO{{O}^{-}}}{\mathop{|}}}\,\]
done
clear
B)
\[C{{H}_{3}}CO{{O}^{-}}\]
done
clear
C)
done
clear
D)
\[AsO_{4}^{3-}\]
done
clear
View Answer play_arrow
question_answer 29) The heat of hydrogenation of \[C{{H}_{2}}=C{{H}_{2}}\]is 30 kcal/mol, what is the heat of hydrogenation of 1, 3 butadiene?
A)
63 kcal/mol
done
clear
B)
57 kcal/mol
done
clear
C)
60 kcal/mol
done
clear
D)
30 kcal/mol
done
clear
View Answer play_arrow
question_answer 30) \[C{{l}_{2}}+{{H}_{2}}S2HCl+S.\] In this chemicals reaction changes in oxidation number of sulphur is
A)
0 to 2
done
clear
B)
-2 to 0
done
clear
C)
2 to 0
done
clear
D)
-2 to -1
done
clear
View Answer play_arrow
question_answer 31) The pH of water is 7. A substance dissolve in water then pH increase pH = 13 substance Y a salt is
A)
strong acid and strong base
done
clear
B)
strong acid and weak base
done
clear
C)
weak acid and weak base
done
clear
D)
weak acid and strong base
done
clear
View Answer play_arrow
question_answer 32) The false name of NaCI is
A)
Sodium chloride
done
clear
B)
Globar salt
done
clear
C)
Rock salt
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) Which statement is incorrect of AgCl?
A)
AgCl is the more soluble than KI
done
clear
B)
AgCl gives precipitate
done
clear
C)
AgCl is less soluble in water
done
clear
D)
Agl is less soluble than AgCl
done
clear
View Answer play_arrow
question_answer 34) The magnetic moment of complex \[Kn\,\,[Mn{{F}_{6}}]\] is 4.9 BM. What is the oxidation number of Mn and what is the value of n?
A)
\[M\,(\,V),\,\,n=1\]
done
clear
B)
\[Mn\,(II),\,\,n=4\]
done
clear
C)
\[M\,(III),\,\,n=3\]
done
clear
D)
\[Mn\,(IV),\,\,n=2\]
done
clear
View Answer play_arrow
question_answer 35) In which of the following is para-uranium element?
A)
Po, Fm, Md
done
clear
B)
Bk, Cf, Am
done
clear
C)
TM, Nd, Pm
done
clear
D)
Th, Np, Pu
done
clear
View Answer play_arrow
question_answer 36) The compound in which underlined carbon uses only its\[s{{p}^{3}}\]hybrid orbitals for bond formation is
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 37) How many numbers of possible stereoisomers are there of 2, 3, 4 trichloro pentanoic acid?
A)
8
done
clear
B)
12
done
clear
C)
16
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 38) State the monomer of Teflon
A)
\[C{{F}_{2}}=C{{F}_{2}}\]
done
clear
B)
\[C{{H}_{2}}=CH\cdot Cl\]
done
clear
C)
\[C{{H}_{2}}=\underset{Cl}{\mathop{\underset{|}{\mathop{C}}\,}}\,-CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CH\cdot CN\]
done
clear
View Answer play_arrow
question_answer 39) Number of unpaired electrons in \[{{O}_{2}}\] molecule is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 40) The minimum configuration of an element is \[4{{s}^{1}},\,3{{d}^{10}}\] possible element is
A)
element of a group 10th
done
clear
B)
a metal
done
clear
C)
a non-metal
done
clear
D)
liquid at \[2981{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 41) An alkyl halide by the formation of its Grignard reagent and heating with water yields propane. What is the original alkyl halide?
A)
Methyl iodide
done
clear
B)
Ethyl iodide
done
clear
C)
Ethyl bromide
done
clear
D)
Propyl bromide
done
clear
View Answer play_arrow
question_answer 42) Most acidic is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 43) Addition of\[B{{r}_{2}}\]on\[C{{H}_{2}}=CH-C{{H}_{2}}-C{{H}_{3}}\] gives
A)
\[Br-C{{H}_{2}}-\overset{Br}{\mathop{\overset{|}{\mathop{CH}}\,}}\,-CH-C{{H}_{3}}\]
done
clear
B)
\[Br-C{{H}_{2}}-C{{H}_{2}}-\overset{Br}{\mathop{\overset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
C)
\[B{{r}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\underset{Br}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
D)
\[Br-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\underset{Br}{\mathop{\underset{|}{\mathop{C{{H}_{2}}}}\,}}\,\]
done
clear
View Answer play_arrow
question_answer 44) The following data are for the decomposition of ammonium nitrite in aqueous solution Vol. of \[{{N}_{2}}\]in cc Time (min.) 6.55 10 9.00 15 11.40 20 13.65 25 35.65 \[\infty \] The order of reaction is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 45) Which Nernst equation is true to find out the potential of non-standard electrochemical cell from the following? \[Fe(s)|F{{e}^{2+}}(aq\,XM)|\,|\,{{I}^{-}}(aq){{I}_{2}}(s)Pt\]
A)
\[{{E}_{cell}}=E_{cell}^{\text{o}}-\frac{0.592}{n}{{\log }_{10}}[F{{e}^{2+}}]\,{{[{{I}^{-}}]}^{2}}\]
done
clear
B)
\[{{E}_{cell}}=E_{cell}^{\text{o}}-\frac{0.0592}{n}{{\log }_{10}}[F{{e}^{2+}}]\,\,{{[{{I}^{-}}]}^{2}}\]
done
clear
C)
\[{{E}_{cell}}=E_{cell}^{\text{o}}-\frac{0.0592}{n}{{\log }_{10}}[F{{e}^{2+}}]\,\,[{{I}^{-}}]\]
done
clear
D)
\[{{E}_{cell}}=E_{cell}^{\text{o}}-\frac{0.0592}{n}{{\log }_{10}}\frac{[F{{e}^{2+}}]\,\,{{[{{I}^{-}}]}^{2}}}{[Fe]\,\,[{{I}_{2}}]}\]
done
clear
View Answer play_arrow
question_answer 46) Half-life of radioactive substance is 4 days. Amount of the substance decayed in two days is
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\left( 1-\frac{1}{\sqrt{2}} \right){{N}_{0}}\]
done
clear
C)
\[20\,%\]
done
clear
D)
\[\frac{1}{8}\]
done
clear
View Answer play_arrow
question_answer 47) \[{{S}_{N}}2\]mechanism is involved in the following substitution
A)
\[C{{H}_{3}}-C{{H}_{2}}-Cl+O{{H}^{-}}\]
done
clear
B)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{Cl}{\mathop{\overset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{C{{H}_{3}}}{\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-C{{H}_{3}}+O{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 48) On oxidation of organic compound A with \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] and \[{{H}_{2}}S{{O}_{4}}\] gives compound B, which on reduction with \[{{H}_{2}}\] in presence of Ni catalyst gives ethyl alcohol. Give the name of compound A.
A)
Ethanal
done
clear
B)
Ethanol
done
clear
C)
Ethanoic acid
done
clear
D)
Ethene
done
clear
View Answer play_arrow
question_answer 49) Cu have the fee unit cell and length is 362 pm, what will be density to copper?
A)
\[6.29g\text{/}c{{m}^{3}}\]
done
clear
B)
\[2.23\text{ }g\text{/}c{{m}^{3}}\]
done
clear
C)
\[4.45g\text{/}c{{m}^{3}}\]
done
clear
D)
\[8.92\text{ }g\text{/}c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 50) Which of the following configuration is correct for the nitrogen?
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{2}}x\,2p{{y}^{1}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{2}}x\,2p{{y}^{1}}z\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2p{{x}^{1}}p{{y}^{1}}2{{p}^{1}}z\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{3}}x\]
done
clear
View Answer play_arrow
question_answer 51) Tautonym is
A)
unscientific explanation of a phenomenon
done
clear
B)
common name used as scientific name
done
clear
C)
non-latinised name
done
clear
D)
same name for genus and species
done
clear
View Answer play_arrow
question_answer 52) Plasmid is
A)
fungus
done
clear
B)
plastid
done
clear
C)
part of plasma membrane
done
clear
D)
extra chromosomal DNA in bacterial cell
done
clear
View Answer play_arrow
question_answer 53) Viruses that infect bacteria, multiply and cause their lysis are
A)
lysozymes
done
clear
B)
lipolytic
done
clear
C)
lytic
done
clear
D)
lysogenic
done
clear
View Answer play_arrow
question_answer 54) Gymnosperms are naked seeded plants because there is no
A)
fruit
done
clear
B)
ovule
done
clear
C)
fertilization
done
clear
D)
ovary and fruit
done
clear
View Answer play_arrow
question_answer 55) Which of the following is an exclusive character of class-Mammalia?
A)
Homoiothermy
done
clear
B)
Internal fertilization
done
clear
C)
Presence of four chambered heart
done
clear
D)
Presence of muscular diaphragm
done
clear
View Answer play_arrow
question_answer 56) Colchicine producing plant belongs to family
A)
Liliaceae
done
clear
B)
Rubiaceae
done
clear
C)
Malvaceae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 57) Diadelphous condition is found in
A)
Rosaceae
done
clear
B)
Papilionaceae
done
clear
C)
Leguminosae
done
clear
D)
Cucurbitaceae
done
clear
View Answer play_arrow
question_answer 58) Growth rings are formed due to activity of
A)
extrastelar cambium
done
clear
B)
intrastelar cambium
done
clear
C)
interstelar cambium
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 59) Myelinated nerve fibres are white coloured because of
A)
chromidial substance
done
clear
B)
neurolemma
done
clear
C)
myelin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 60) In which of the following tissues is the matrix not a product of synthesis of its cells?
A)
Muscular tissue
done
clear
B)
Osseous tissue
done
clear
C)
Loose connective tissue
done
clear
D)
Adipose tissue
done
clear
View Answer play_arrow
question_answer 61) There are special proteins that helps to open up DNA double helix in the front of replication fork. These proteins are
A)
DNA ligase
done
clear
B)
DNA gyrase
done
clear
C)
DNA polymerase
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 62) Elaioplast store
A)
starch
done
clear
B)
proteins
done
clear
C)
fats
done
clear
D)
essential amino acids
done
clear
View Answer play_arrow
question_answer 63) Which of the following is the site of lipid synthesis?
A)
Rough ER
done
clear
B)
Smooth ER
done
clear
C)
Golgi bodies
done
clear
D)
Ribosomes
done
clear
View Answer play_arrow
question_answer 64) Which of the following statement is wrong?
A)
Sucrose is a diasaccharide
done
clear
B)
Cellulose is a polysaccharide
done
clear
C)
Glycine is sulphur containing amino acid
done
clear
D)
Uracil is a pyrimidine
done
clear
View Answer play_arrow
question_answer 65) Phenomenon of crossing over in diploid organism is responsible for
A)
linkage between genes
done
clear
B)
recombination between linked genes
done
clear
C)
segregation between genes
done
clear
D)
dominance of genes
done
clear
View Answer play_arrow
question_answer 66) Nitrates are converted into nitrogen by
A)
nitrogen fixing bacteria
done
clear
B)
sulphur fixing bacteria
done
clear
C)
denitrifying bacteria
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 67) During oxidative phosphorylation, the net gain of ATP is
A)
40
done
clear
B)
38
done
clear
C)
34
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 68) Closure of lid of pitcher in pitcher plant is
A)
tropic movement
done
clear
B)
parotonic movement
done
clear
C)
turgor movement
done
clear
D)
autonomous movement
done
clear
View Answer play_arrow
question_answer 69) What is the principle cation in human blood?
A)
Potassium
done
clear
B)
Sodium
done
clear
C)
Calcium
done
clear
D)
Maganese
done
clear
View Answer play_arrow
question_answer 70) Which one of the following body functions is not performed by kidneys?
A)
Excretion
done
clear
B)
Osmoregulation
done
clear
C)
Regulation of blood volume
done
clear
D)
Destruction of dead blood corpuscles
done
clear
View Answer play_arrow
question_answer 71) Endocrine glands are
A)
ductless glands whose secretions pour directly into blood
done
clear
B)
have ducts and pour their secretion into blood directly
done
clear
C)
have ducts which straight way pour secretions into target organs
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 72) Vegetative fertilization leading to the formation of endosperm refers to
A)
fusion of male gamete with diploid secondary nucleus
done
clear
B)
fusion of female gamete with diploid secondary nucleus
done
clear
C)
fusion of two diploid vegetative cells
done
clear
D)
fusion of two male gametes
done
clear
View Answer play_arrow
question_answer 73) Regeneration of tail in lizard is an example of
A)
epimorphosis
done
clear
B)
morphollaxis
done
clear
C)
heteromorphosis
done
clear
D)
parthenogenesis
done
clear
View Answer play_arrow
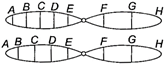
question_answer 74)
Given below is a representation of a kind of chromosomal mutation. What is the kind of mutation represented?
A)
Deletion
done
clear
B)
Duplication
done
clear
C)
Inversion
done
clear
D)
Reciprocal translocation
done
clear
View Answer play_arrow
question_answer 75) The maximum growth rate occurs in
A)
stationary phase
done
clear
B)
senescent phase
done
clear
C)
lag phase
done
clear
D)
exponential phase
done
clear
View Answer play_arrow
question_answer 76) Directions: Select the one which is different from the other three responses. Select the one which is different from the other three responses.
A)
Flute
done
clear
B)
Violin
done
clear
C)
Guitar
done
clear
D)
Sitar
done
clear
View Answer play_arrow
question_answer 77)
Directions: Which one of the given responses would be a meaningful order of the following words in ascending order? 1. Neonate 2. Child 3. Infant 4. Embryo
A)
1, 3, 2, 4
done
clear
B)
4, 1, 3, 2
done
clear
C)
4, 3, 1, 2
done
clear
D)
3, 1, 4, 2
done
clear
View Answer play_arrow
question_answer 78) Find the wrong number in the given series 16, 22, 30, 45,52,66
A)
30
done
clear
B)
45
done
clear
C)
52
done
clear
D)
66
done
clear
View Answer play_arrow
question_answer 79) In a row of boys, if A who is tenth from the left and B who is ninth from the right interchange their positions. Now, A becomes fifteenth from the left. How many boys are there in the row?
A)
23
done
clear
B)
27
done
clear
C)
28
done
clear
D)
31
done
clear
View Answer play_arrow
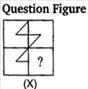
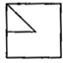
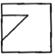
question_answer 80)
Which answer figure will complete the pattern of given question figure?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 81) Which one among the following countries has the lowest GDP per captita?
A)
China
done
clear
B)
India
done
clear
C)
Indonesia
done
clear
D)
Sri Lanka
done
clear
View Answer play_arrow
question_answer 82) The Constitution of India was adopted on
A)
26th January, 1950
done
clear
B)
26th January, 1949
done
clear
C)
26th November, 1949
done
clear
D)
15th August, 1947
done
clear
View Answer play_arrow
question_answer 83) How many states and union territories are there in India?
A)
25 states and 7 union territories
done
clear
B)
28 states and 7 union territories (including National capital territory II)
done
clear
C)
24 states and 6 union territory
done
clear
D)
None of the Above
done
clear
View Answer play_arrow
question_answer 84) All of the following statements regarding the Indus Valley civilization are correct except
A)
The Indus Valley civilization was an advanced urban civilization
done
clear
B)
Iron was not known to the people
done
clear
C)
It is difficult to say which race the people belonged
done
clear
D)
The people know nothing about agriculture
done
clear
View Answer play_arrow
question_answer 85) The principle that disguishes Jainism from Buddhism is the
A)
practice of the eight-fold path
done
clear
B)
rejection of the infallibility of the Vedas
done
clear
C)
attribution of a soul to all beings and things
done
clear
D)
belief in rebirth
done
clear
View Answer play_arrow
question_answer 86) Light year is unit of
A)
time
done
clear
B)
speed of light
done
clear
C)
distance
done
clear
D)
mass
done
clear
View Answer play_arrow
question_answer 87) Pick out the only vector quantity
A)
pressure
done
clear
B)
impulse
done
clear
C)
gravitational
done
clear
D)
coefficient of friction potential
done
clear
View Answer play_arrow
question_answer 88) The computers processor consists of the following parts
A)
CPU and Main Memory
done
clear
B)
Hard Disk and Floppy Drive
done
clear
C)
Main Memory and Storage
done
clear
D)
Operating System and Applications
done
clear
View Answer play_arrow
question_answer 89) Soft copy is an intangible output, so then what is a hard copy?
A)
The physical parts of the computer
done
clear
B)
The printed parts of the computer
done
clear
C)
The printed output
done
clear
D)
The physical output device
done
clear
View Answer play_arrow
question_answer 90) Which of the following is considered a hotspot of biodiversity in India?
A)
Aravali hills
done
clear
B)
Western Ghats
done
clear
C)
Indo-gangetic plain
done
clear
D)
Eastern Ghats
done
clear
View Answer play_arrow
question_answer 91) First National park developed in India is
A)
Gir
done
clear
B)
Kaziranga
done
clear
C)
Jim Corbett
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 92) The First-Earth Sumti was held at
A)
Buenos Aires
done
clear
B)
Rio de Janeiro
done
clear
C)
Dar-es-salam
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 93) What is the name of Kalhanas book?
A)
Arthashastra
done
clear
B)
Indica
done
clear
C)
Purana
done
clear
D)
Rajtarangini
done
clear
View Answer play_arrow
question_answer 94) 1st Tuesday of may is observed as
A)
Global Family Day
done
clear
B)
World Asthma Day
done
clear
C)
World Mothers Day
done
clear
D)
International Human Solidarity Day
done
clear
View Answer play_arrow
question_answer 95) With which game does Dovis cup is associated?
A)
Hockey
done
clear
B)
Table Tennis
done
clear
C)
Lawn Tennis
done
clear
D)
Polo
done
clear
View Answer play_arrow
question_answer 96) Which is the greatest out of the following numbers?
A)
\[{{(2+2+2)}^{2}}\]
done
clear
B)
\[{{[{{(2+2)}^{2}}]}^{2}}\]
done
clear
C)
\[{{(2\times 2\times 2)}^{2}}\]
done
clear
D)
\[{{4}^{3}}\]
done
clear
View Answer play_arrow
question_answer 97) If after the payment of \[\frac{3}{4}\] of a loan Rs. 500 still remain to pay, what is whole amount of the loan?
A)
Rs. 2000
done
clear
B)
Rs. 2100
done
clear
C)
Rs. 1700
done
clear
D)
Rs. 1500
done
clear
View Answer play_arrow
question_answer 98) Directions: In given sentence to find out whether there is any grammatical error in it. The error, if any, will be in one part of the sentence. The number of that part is the answer. If there is no error, the answer is i.e., No error. (Ignore the errors of punctuation, if any)
A)
I am grateful to you and all your friends
done
clear
B)
/ for they showed sympathy
done
clear
C)
/ and kindness towards me.
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 99) Directions: In each sentence below a word is printed in bold. Below each sentence, four words/ group of words are suggested, one of which can replace the bold word, without changing the meaning of the sentence. Find out the appropriate word/ group of words in each case. His inflexible attitude is the root cause of most of his problems.
A)
negative
done
clear
B)
nasty
done
clear
C)
hesitant
done
clear
D)
rigid
done
clear
View Answer play_arrow
question_answer 100) Directions: In each question below three words are given numbered as (a), (b) and (c). One of these three words may be wrongly spelt. Find out the word which is wrongly spelt, if there is any. The number of that word is your answer. If all the words are correctly spelt, mark, i.e., All correct as your answer.
A)
Adventure
done
clear
B)
Demonstration
done
clear
C)
In no sent
done
clear
D)
All correct
done
clear
View Answer play_arrow