A) one \[N-O\] bond is shorter than the other two equal bonds
B) one \[N-O\] bond is longer than the other two equal bonds
C) all the \[N-O\] bonds are equal in length
D) all the \[N-O\] bonds are unequal in length
E) None of the above
Correct Answer: C
Solution :
The resonating structure of nitrate ion \[(NO_{3}^{-})\]is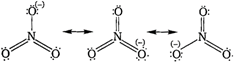 As a result of resonance, the bond lengths of\[N-O\] bond in a molecule becomes equal.
As a result of resonance, the bond lengths of\[N-O\] bond in a molecule becomes equal.
You need to login to perform this action.
You will be redirected in
3 sec
