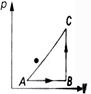
A) 34 J
B) 70 J
C) 84 J
D) 134 J
E) None of these
Correct Answer: A
Solution :
From the first law of thermodynamics, if an amount of heat Q is given to a system, a part of it will be used in increasing the internal energy \[(\Delta U)\]of the system and rest in doing work (W) by the system. Therefore, \[Q=\Delta U+W\] \[\Rightarrow \] \[\Delta U=Q-W\] Given, \[Q=20\,cal=20\times 4.2J=84\,J\] \[W=50\,J\] \[\therefore \] \[\Delta U=(84-50)J=34\,J\]You need to login to perform this action.
You will be redirected in
3 sec
