A) Hund's rule
B) Aufbau principle
C) Uncertainty principle
D) Pauli's exclusion principle
Correct Answer: D
Solution :
According to Pauli's principal, "No two electrons with in the same atom can have the same values for all the four quantum numbers".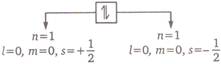 No two electrons can have same position and energy in an atom. If two electrons in an atom have same value of n,( and m, the value of their spin quantum number(s) will be different. Thus, if one electron has
No two electrons can have same position and energy in an atom. If two electrons in an atom have same value of n,( and m, the value of their spin quantum number(s) will be different. Thus, if one electron has You need to login to perform this action.
You will be redirected in
3 sec
