A) \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{2}}-C{{H}_{3}}\]
B) \[{{(C{{H}_{3}})}_{3}}C-C{{H}_{2}}OH\]
C) \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
D) \[{{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}OH\]
Correct Answer: D
Solution :
: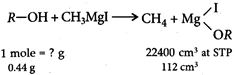 Mass of alcohol \[=\frac{0.44\times 22,400}{112}=88g\] Alcohol reacts with PCC to give a carbonyl compound which answers silver mirror test. Therefore, alcohol must be primary alcohol which on oxidation with PCC gives aldehyde; (carbonyl compound). Therefore, either or is correct. Out of these has the mass 88.
Mass of alcohol \[=\frac{0.44\times 22,400}{112}=88g\] Alcohol reacts with PCC to give a carbonyl compound which answers silver mirror test. Therefore, alcohol must be primary alcohol which on oxidation with PCC gives aldehyde; (carbonyl compound). Therefore, either or is correct. Out of these has the mass 88.
You need to login to perform this action.
You will be redirected in
3 sec
